cms/lib/NY01001456/Centricity/domain/535/silent tea parties/ans
advertisement

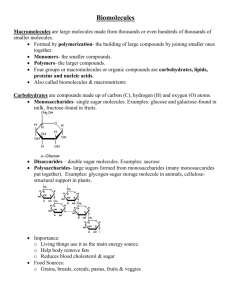
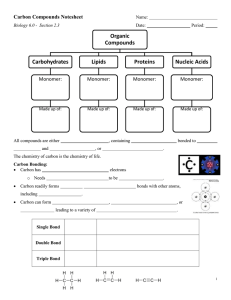
BiochemistryR Silent Tea Party Name_______________ 1. What are macromolecules? Four main classes of large biological molecules (carbohydrates, lipids, proteins, nucleic acids) made up of many smaller molecules and atoms. 2. What are monomers? small chemical unit that can join together with other small units to form larger units called polymer 3. What are polymers? Large compounds formed from combinations of many monomers. 4. What are organic compounds? Compounds that contain carbon atoms and are found in living things. They usually contain both Carbon and Hydrogen. 5. What are inorganic compounds? Compounds that do not contain both carbon and hydrogen; many are also essential to life. 6. What are polysaccharides? large carbohydrate molecules composed of many smaller units, linked together in complex arrangements 7. What are disaccharides? sugar molecules with only two monomers 8. What are monosaccharides simple sugar molecules that are a building block for polysaccharides(glucose and fructose are examples) 9. What are fatty acids? One of the components of lipids 10. What are saturated fats? A fat that is solid at room temperature, found in animal fats such as lard and butter. All of its carbon to carbon bonds are single. Too much of this can increase the chance of cardiovascular disease 11. What are unsaturated fats? A fat that contains fewer numbers of hydrogen (less stored energy) and is liquid at room temperature, found in vegetable oils, nuts, and seeds. Atleast one of its carbon to carbon bonds is double or triple. 12. What are proteins? macromolecules containing carbon, hydrogen, oxygen and nitrogen; usually made of more than one polypeptide chain. 13. What are amino acids? building blocks (monomers) of proteins; twenty kinds 14. What are Reactants? Substances that takes part in and undergoes change during a reaction. 15. What are Products? The substances that result from a recombination of atoms. 16. What are Isomers? Two or more compounds with the same molecular formula but a different arrangement of atoms in the molecule and different properties. 17. What is Dehydration Synthesis? Covalently joining two monomers together. Water is released as a result. 18. What is Hydrolysis? Breaking a polymer into shorter strands by adding water. 19. What are the functions of carbohydrates? Provides short term energy, long term energy and structure in plants 20. What are Complex carbohydrates? Polysaccharide- Starch and cellulose 21. What is the molecular formula for glucose? C6 H12O6 22. What are Polypeptides? Polymer of amino acids 23. What is the Function of Proteins? Build and repair tissue, structures, enzymes, membrane transport, immunity 24. What is denaturation? A change in shape of a protein due to high temperatures or extreme pHs, causing protein to stop working 25. What is starch? a polysaccharide found in plants to store energy long term 26. What is glycogen? a polysaccharide found in animals to store energy long term 27. What is cellulose? a polysaccharide found in plants to provide structural support(cell wall) 28. What is phospholipid? a lipid with two fatty acid tails and a polar phosphate head. It's the molecule that makes up most of the cell membrane. 29. What is peptide bond? the bond between two amino acids 30. What is the molecular formula for a disaccharide? C12 H 22O 11 31. What is the function of lipids? Long term energy storage and phospholipids make up the cell membrane