AP Chemistry – Density Lab
advertisement

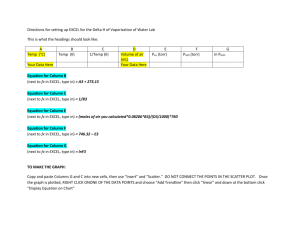
AP Chemistry – Density Lab Goal: Find the density of a metal sample as precisely as possible (at least 2 significant digits). For aluminum: Find the density using the method you choose. You have all standard lab equipment to choose from, including but not limited to: various sizes of graduated cylinders, beakers, and flasks; analytical balances; rulers; and calipers. If you choose the water displacement method, be sure to ease the samples into the container you choose to avoid breakage. You will find % error for aluminum in two different methods: 1. Compare your personal findings with the accepted value in the CRC Handbook of Chemistry and Physics. 2. Compare your personal findings with the slope of a graph obtained by plotting the class data. For copper: Use the same procedure as for the aluminum. You will find % error for copper in two different methods: 1. Compare your personal findings with the accepted value in the CRC Handbook 2. Compare your personal findings with the mean of the class data. Class data: Aluminum Mass Volume Copper Mass Volume In your error analysis section, be sure to discuss the factor(s) that limited the amount of precision in your answers. Also, be sure to discuss the advantages and disadvantages to the determination method you chose over other methods. And, be sure to discuss the different % error methods used for each sample and why you believe one is better than the other (or the same). At what point was a graph useful? Not useful? The error analysis section should be the largest section of your report! To calculate the density on Excel, enter the mass and volume data in separate columns. Then, in an empty column to the right, click in a box and enter the formula. To enter a formula, type = and then the names of the boxes you want and the mathematical operations (example: =d4/e4). To repeat the formula with the next set of data, simply click on the formula box and drag down to highlight the entire column. Then, select “Fill” and “Down”. The formula will be copied, but advanced by one row automatically. You can set the decimal places by right clicking, and select “Format Cell”. Then, select “Number”. Since Excel will not find significant digits, you will have to set the number of decimal places for each one individually. Submit your formal lab report on Canvas electronically. Be sure to include your graph and pictures of all your collected data. How to Make a Linear Plot using Microsoft Excel 2010 To show a linear relationship using Excel, such as density, complete the following steps: 1. Enter a set of values in column A. 2. Enter a set of values in column B. 3. Set the data range by selecting all the data. (Click in a corner and drag the mouse until all boxes are selected. Do NOT include the titles.) 4. Click the Insert tab. In the charts menu, click on Scatter. Select the first option, scatter with only markers. 5. Right click on the graph, and select Select Data. Click on Series1 (on the left) if it is not already selected, and press the Edit button just above it. Make sure the proper X and Y values are selected. (If you put Y as column A and X as column B, this will be done automatically. If X and Y are backwards, re-select the values on this window.) For example, on a density plot the mass should be on the Y-axis, and volume on the X-axis. 6. At the top, there will be a green area labeled Chart Tools. Select the Layout tab. Click on Axis Titles in the Labels submenu. Enter the titles for both axes. Don’t forget the units! (Use rotated on the vertical axis.) Also, select Chart Title to add a title for the entire chart. 7. To draw a straight line thru the data, right click on a data point, and select "Add Trendline". 8. Select Linear regression. If the plot is to go thru the origin, check the "Set Intercept" box, and enter 0 in the box. To show the equation of the line (y=mx +b), check the "Show Equation" box. If you want to discuss the R2 value, select it as well. 9. Move objects around so they are clearly visible. Make any final adjustments to make the graph clear and readable. You can adjust the axis properties by right clicking on the axis, and select Format Axis. 10. Be sure the plot takes up most of the space. Right click on the chart, and select Format Chart Area. Then, select Size. Here is a sample density plot from class data obtained by an AP Chemistry class. To calculate the density column, enter the formula "= B2/A2" to divide the mass by the volume in the first box (C2). Click on that box, and drag down to the bottom of the data. On the Home screen, at the far right in the Editing menu, select "Fill" and "Down". The spreadsheet will automatically change the row numbers in the formula for each entry. Density of Aluminum 100.0 y = 2.805x Mass (g) 80.0 60.0 40.0 Series1 20.0 Linear (Series1) 0.0 0.0 10.0 20.0 Volume (mL) 30.0 40.0