(一)目的 1. To determine Avogadro's number using molecular
advertisement

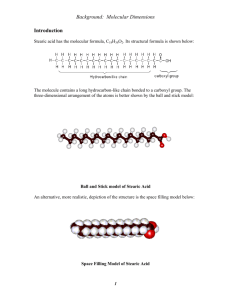
亞佛加厥常數之測定 (一)目的 1. To determine Avogadro’s number using molecular monolayer techniques 2. To find the number of molecules in a monolayer 3. To find the moles of stearic acid in a monolayer (二)前言 Avogadro's number , considered one of the few fundamental constants that must be determined independently, is the number of atoms or molecules present in a sample with a mass, expressed in grams, numerically equal to its atomic or molecular mass expressed in amu's or Daltons. It is 12 12 defined as the number of C atoms in exactly 12 grams of C. (三)實驗原理 Some of the more than twenty different types of experiments used to determine Avogadro's number utilize methods for directly counting the number of molecules present in a sample of a compound with known mass. This is the type of experiment that you will perform. Under appropriately controlled conditions, it is possible to spread a film of a fatty substance only one molecule thick, a monolayer, onto a water surface. The thickness of the monolayer can be calculated from the measured volume of substance required to make the monolayer and the measured area of the surface that is covered. Substances such as stearic acid (C17H35COOH) are called fatty acids due to their presence in many natural fats. Such molecules include two major fragments: A long hydrocarbon chain and a carboxylic acid group. Stearic Acid, C17H35COOH The polar -COOH acid group is attracted to water molecules where the nonpolar hydrocarbon chain is repelled by water molecules. Stearic acid is not soluble in water so to make a solution, it needs to be dissolved in a different solvent. When a solution of stearic acid in a volatile hydrocarbon solvent is placed on water, the stearic acid spreads out on the surface of the water such that each molecule has its polar –COOH group in the water and its nonpolar hydrocarbon chain above the water surface (see Figure 1). The hydrocarbon solvent evaporates quickly, leaving behind a monolayer of stearic acid. Additional aliquots of stearic acid solution will cause the fatty acid molecules to spread and become more compact, adopting a vertical orientation until the entire water surface is covered. This is because each molecule “wants” to have its polar group in the water and the hydrocarbon chain in the air. The fully compact state of the monolayer is shown schematically in Figure 1. Figure 1 Schematic magnified view of a stearic acid monolayer on a water surface. Only a few of the many molecules are shown for clarity. Subsequent addition of stearic acid solution will cause it to float on top of the existing monolayer forming a lens-shaped droplet that is readily visible. The number of drops of stearic acid solution required to form the monolayer can thus be determined. The mass of stearic acid in the monolayer can be calculated from the measured volume and known concentration of the stearic acid solution. The number of moles of stearic acid in the monolayer is determined using its molar mass. We do not directly know the surface area of the water occupied by each stearic acid molecule. However, we can make guesses based on the molecular geometry of stearic acid. If we assume each stearic acid molecule on the surface has a cubical shape with side length s, it then has a height s and 2 occupies an area s . Assuming that there are no spaces between stearic acid molecules in the 2 monolayer, each molecule occupies an area of about 0.21 nm . The total number of stearic acid 2 molecules in the monolayer can be determined from s and the measured area of the monolayer. Avogadro’s number is obtained by dividing the number of molecules of stearic acid in the monolayer by the number of moles of stearic acid in the layer. (四)器材與藥品:每組份量 天平/秤量紙 方格紙 硬脂酸 己烷 塑膠滴管 1支 玻璃滴管 1支 100 mL燒杯 1個 50 mL量筒 1個 錶玻璃 1個 10ml量筒 1個 (五)實驗步驟設計: In this experiment we will determine an experimental value for Avogadro’s number. First, the solution of stearic acid is prepared by dissolving the solid substance in the organic solvent hexane. The solution is then added drop by drop onto the water contained in a watch glass. After each drop is added, the stearic acid molecules spread across the surface of the water forming a single layer, a monolayer. A few seconds after each drop of solution is added, the hexane solvent evaporates and the drop disappears. When enough drops of solution have been added to form a monolayer of stearic acid molecules, one additional drop forms a clear bead or lens. 1. 決定一滴硬脂酸/己烷溶液體積 2. 單層分子膜的產生,利用方格紙測量此單層分子膜面積 3. 計算此單層分子中膜硬脂酸分子數目 (Assuming that there are no spaces between stearic acid molecules 2 in the monolayer, each molecule occupies an area of about 0.21 nm .) -4 4. 計算此單層分子中膜硬脂酸重(concentration of stearic acid solution = 1.5 x 10 g/mL) 5. 計算此單層分子中膜硬脂酸莫耳數 (molar mass of stearic acid =284 g/mol),藉其莫耳數推求亞佛 加厥數 (六)數據紀錄與列式: 組別 組員 組長 (七)問題與討論 22 1. Calculate the mass of carbon in a 1 carat diamond that contains 1.00 x 10 atoms of carbon. 2. What is the mass of one molecule of stearic acid, C17H35COOH, expressed in grams? 3. 本實驗為何選用硬脂酸?該分子結構有何特色? 4. 你認為本實驗的主要誤差來自於?如何改善? 5. 試簡述其他測量亞佛加厥常數之方法及原理?(如電化學測定法、放射性元素衰變法、結晶 學法等)