Apparatus and Materials
advertisement

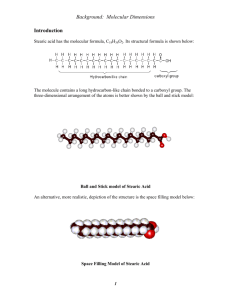
DETERMINING AVOGADROS CONSTANT Introduction Some molecules, such as stearic acid (below), have one end that is attracted to water (hydrophillic) and one end that is repelled by it (hydrophobic). When you put such molecules onto water they can form a monolayer (a layer a single molecule thick), whereby their hydrophillic ends stick into the water and their hydrophobic tails stick up (see diagram right, not to scale!). If we drop a small amount of stearic acid onto water, it will spread out to form a monolayer and we can measure the area this occupies. The monolayer will be circular (it is formed in a circular watch glass) so: 𝐴𝑟𝑒𝑎(𝑚𝑜𝑛𝑜𝑙𝑎𝑦𝑒𝑟) = 𝜋𝑟 2 If we know the cross-sectional (end-on) area of a molecule of stearic acid (this is approximately 2.1x10-15 cm2) we can calculate the number of molecules in our monolayer: 𝑁(𝑠𝑡𝑒𝑎𝑟𝑖𝑐 𝑎𝑐𝑖𝑑 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠) = 𝐴𝑟𝑒𝑎 (𝑚𝑜𝑛𝑜𝑙𝑎𝑦𝑒𝑟) 𝐴𝑟𝑒𝑎 (𝑠𝑡𝑒𝑎𝑟𝑖𝑐 𝑎𝑐𝑖𝑑 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒) If we know the mass of stearic acid used and the relative molecular mass we can work out the number of moles we have: 𝑛(𝑠𝑡𝑒𝑎𝑟𝑖𝑐 𝑎𝑐𝑖𝑑) = 𝑚𝑎𝑠𝑠 𝑢𝑠𝑒𝑑 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 Finally we can combine the number of molecules and the number of moles to work out Avogadro’s constant: 𝐿= 𝑁(𝑠𝑡𝑒𝑎𝑟𝑖𝑐 𝑎𝑐𝑖𝑑 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠) 𝑛(𝑠𝑡𝑒𝑎𝑟𝑖𝑐 𝑎𝑐𝑖𝑑) Apparatus and Materials Pasteur pipette Watch glass Stearic acid dissolved in hexane (0.10 g cm-3) Hexane Propanone Distilled water Washing-up liquid Procedure Calibrating the pipette: 1. Fill a Pasteur pipette with hexane 2. Add the hexane dropwise to measuring cylinder, counting the number of drops required to reach a volume of 1.0 cm3. 3. Repeat steps 1-2 until you obtain consistent drop-counts. 4. Empty the pipette and then ‘squirt’ it until all remaining traces of hexane have completely evaporated. Producing the monolayer: 1. Obtain a watch glass and measure its diameter as accurately as possible. 2. Thoroughly clean the watch glass with detergent and water, rinsing thoroughly with distilled water and then with propanone. 3. Allow the watch glass to fully dry, ensuring you do not touch the inside to avoid contamination. 4. Place the watch glass on a level surface and fill it to the brim with distilled water. 5. Using the calibrated pipette, place a single drop of the hexane-stearic acid solution on the surface of the water. The droplet should rapidly spread out and then disappear within a few seconds. 6. Once the previous droplet has disappeared, add another and wait for it to also disappear.* 7. Repeat step six, counting the drops added until you get a drop that, rather than spreading out, forms a ‘lens’ on the surface, this means that there is a monolayer on the surface and so the drop cannot penetrate. 8. Check that the monolayer is complete by adding another drop at a different location and seeing whether it forms a lens. *If after a few drops you see an oily residue on the surface, your watch glass was not clean enough and you need to start again, washing more thoroughly. Analysis Follow the calculations outlined in the introduction to calculate Avogadro’s constant. You can ask for help if you need it but should challenge yourself to do it without help. How does your calculated value of Avogadro’s constant compare to the literature value? What do you think are the main sources of error in this experiment? How could they be overcome? What assumptions does our method make? Are these valid assumptions?