FM1003 Exam Qs

Give a brief account of the three phases of deglutition (swallowing).
1. Swallowing can be divided into voluntary, pharyngeal and esophageal stages.
When the food is ready for swallowing, it is voluntarily rolled up against the palate by the action of the tongue towards the pharynx. After this the process is completely automatic.
2. The soft palate is pulled upward to close the posterior nares, to prevent reflux of food into the nasal cavities. The palatopharyngeal folds on each side of the pharynx are pulled medially to approximate each other. In this way, these folds form a sagittal slit through which the food must pass into the posterior pharynx.
This slit performs a selective action, allowing food that has been masticated sufficiently to pass with ease. Because this stage of swallowing lasts less than 1 second, any large object is usually impeded too much to pass into the esophagus.
The vocal cords of the larynx are strongly approximated, and the larynx is pulled upward and anteriorly by the neck muscles. These actions, combined with the presence of ligaments that prevent upward movement of the epiglottis, cause the epiglottis to swing backward over the opening of the larynx. All these effects acting together prevent passage of food into the nose and trachea. Most essential is the tight approximation of the vocal cords, but the epiglottis helps to prevent food from ever getting as far as the vocal cords. Destruction of the vocal cords or of the muscles that approximate them can cause strangulation.
The upward movement of the larynx also pulls up and enlarges the opening to the esophagus. At the same time, the upper 3 to 4 centimeters of the esophageal muscular wall, called the upper esophageal sphincter (also called the pharyngoesophageal sphincter) relaxes, thus allowing food to move easily and freely from the posterior pharynx into the upper esophagus. Between swallows, this sphincter remains strongly contracted, thereby preventing air from going into the esophagus during respiration. The upward movement of the larynx also lifts the glottis out of the main stream of food flow, so that the food mainly passes on each side of the epiglottis rather than over its surface; this adds still another protection against entry of food into the trachea.
Once the larynx is raised and the pharyngoesophageal sphincter becomes relaxed, the entire muscular wall of the pharynx contracts, beginning in the superior part of the pharynx, then spreading downward over the middle and inferior pharyngeal areas, which propels the food by peristalsis into the esophagus.
3.
The esophagus normally exhibits two types of peristaltic movements: primary peristalsis and secondary peristalsis . Primary peristalsis is simply continuation of the peristaltic wave that begins in the pharynx and spreads into the esophagus during the pharyngeal stage of swallowing. This wave passes all the way from the pharynx to the stomach in about 8 to 10 seconds. Food swallowed by a person who is in the upright position is usually transmitted to the lower end of the esophagus even more rapidly than the peristaltic wave itself, in about 5 to 8 seconds, because of the additional effect of gravity pulling the food downward. If the primary peristaltic wave fails to move into the stomach all the food that has entered the esophagus, secondary peristaltic waves result from distention of the
esophagus itself by the retained food; these waves continue until all the food has emptied into the stomach. The secondary peristaltic waves are initiated partly by intrinsic neural circuits in the myenteric nervous system and partly by reflexes that begin in the pharynx and are then transmitted upward through vagal afferent fibers to the medulla and back again to the esophagus through glossopharyngeal and vagal efferent nerve fibers.
Describe the formation of saliva. How is the basal salivary composition modified?
Indicate using a diagram the mechanism by which the acinar cells of the salivary gland secrete fluid. What changes occur in this secretion in the salivary ducts?
Describe the electrolyte composition of saliva, and compare with the electrolytes found in plasma.
The principal glands of salivation are the parotid, submandibular, and sublingual glands; in addition, there are many very small buccal glands. Daily secretion of saliva normally ranges between 800 and 1500 milliliters. Saliva contains two major types of protein secretion: (1) a serous secretion that contains ptyalin (an
α-amylase), which is an enzyme for digesting starches, and (2) mucus secretion that contains mucin for lubricating and for surface protective purposes. The parotid glands secrete almost entirely the serous type of secretion, while the submandibular and sublingual glands secrete both serous secretion and mucus.
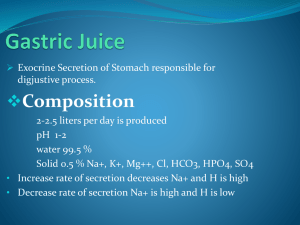
The buccal glands secrete only mucus. Saliva has a pH between 6.0 and 7.0, a favorable range for the digestive action of ptyalin. Saliva contains especially large quantities of potassium and bicarbonate ions. Conversely, the concentrations of both sodium and chloride ions are several times less in saliva than in plasma.
Salivary secretion is a two-stage operation: the first stage involves the acini, and the second, the salivary ducts. The acini secrete a primary secretion that contains ptyalin and/or mucin in a solution of ions in concentrations not greatly different from those of typical extracellular fluid. As the primary secretion flows through the ducts, two major active transport processes take place that markedly modify the ionic composition of the fluid in the saliva. First, sodium ions are actively reabsorbed from all the salivary ducts and potassium ions are actively secreted in exchange for the sodium. Therefore, the sodium ion concentration of the saliva becomes greatly reduced, whereas the potassium ion concentration becomes increased. However, there is excess sodium reabsorption over potassium secretion, and this creates electrical negativity of about -70 millivolts in the salivary ducts; this in turn causes chloride ions to be reabsorbed passively.
Therefore, the chloride ion concentration in the salivary fluid falls to a very low level, matching the ductal decrease in sodium ion concentration. Second, bicarbonate ions are secreted by the ductal epithelium into the lumen of the duct.
This is at least partly caused by passive exchange of bicarbonate for chloride ions, but it may also result partly from an active secretory process. The net result of these transport processes is that under resting conditions, the concentrations of sodium and chloride ions in the saliva are only about 15 mEq/L each, about one seventh to one tenth their concentrations in plasma. Conversely, the
concentration of potassium ions is about 30 mEq/L, seven times as great as in plasma; and the concentration of bicarbonate ions is 50 to 70 mEq/L, about two to three times that of plasma.
What are the functions of saliva?
The water and mucin form a lubricant that moistens food, helps swallowing and aids in speech. In addition, the water facilitates taste by partially dissolving ingested material. Saliva maintains the oral epithelium by keeping it hydrated nad stopping bacteria growth. It also prevents dental caries. The digestion of CHO begins in the mouth by the secretion of salivary alpha amylase, though it is not very effective.
By what mechanisms is salivation controlled?
Increases in salivary flow may result from stimulation of receptors in the mouth, pharynx and oesophagus. Food in the mouth stimulates taste receptors and irritates the oral mucosa while chewing activates a variety of mechanoreceptors such as pressure receptors adjacent to teeth and the muscle spindles of masticatory muscles. These increases in salivation are controlled solely by nervous activity. The salivatory centres also receive impulses from higher centers in the brain and so the sight, smell or thought of food may cause salivation whereas sleep, fatigue, dehydration and fear can inhibit it. Parasympathetic stimulation causes a prolonged copious secretion accompanied by vasodilation.
Sympathetic stimulation on the other hand produces a thick, mucous saliva which is accompanied by vasoconstriction.
Describe the role of the pancreas in regulation of blood glucose concentration.
The pancreas is responsible for insulin secretion into the blood from the Islets of
Langerhans. Within seconds after insulin binds with its membrane receptors, the membranes of about 80 per cent of the body's cells markedly increase their uptake of glucose . This is especially true of muscle cells and adipose cells but is not true of most neurons in the brain. The increased glucose transported into the cells is immediately phosphorylated and becomes a substrate for all the usual carbohydrate metabolic functions. The increased glucose transport is believed to result from translocation of multiple intracellular vesicles to the cell membranes; these vesicles carry in their own membranes multiple molecules of glucose transport proteins, which bind with the cell membrane and facilitate glucose uptake into the cells. When insulin is no longer available, these vesicles separate from the cell membrane within about 3 to 5 minutes and move back to the cell interior to be used again and again as needed. When insulin is secreted into the blood, it circulates almost entirely in an unbound form; it has a plasma half-life that averages only about 6 minutes, so that it is mainly cleared from the circulation within 10 to 15 minutes. Except for that portion of the insulin that combines with receptors in the target cells, the remainder is degraded by the enzyme insulinase mainly in the liver, to a lesser extent in the kidneys and muscles, and slightly in most other tissues. This rapid removal from the plasma is
important, because, at times, it is as important to turn off rapidly as to turn on the control functions of insulin.
What is the role of the vagus nerve in gastric acid secretion?
Gastric acid secretion can be divided into three stages- cephalic, gastric and intestinal. In these phases, there is both endocrine and nervous control of gastric acid secretion and they are to some degree interlinked. The vagus nerve endings when stimulated secrete acetylcholine. This then acts on the muscuranic receptors to raise the concentrations of intracellular calcium which then activates the hydrogen-potassium ATPase. This is particularily important in the cephalic phase when it causes up to 20% of the secretion in response to sight, smell or taste. It then participates in the gastric phase in combination with gastrin and the local enteric reflexes. This stage is responsible for 70% of gastric secretion.
Gastrin is a weak stimulator of secretion and requires the oxyntic cells to be preconditioned by the combined effects of histamine and acetylcholine. The agents can each cause the release of each other and when they act together their actions are synergistic.
In digestion what is the importance of the secretions of the exocrine pancreas?
With the appropriate stimulus the pancreas secretes hydrolytic enzymes that act on the major group of nutrients. The digestive secretions are initially formed in acini that are similar to those in salivary glands, pass through ducts and enter the duodenum. There is a high level of bicarbonate in these secretions the function of which is to neutralize the acidity of the chime from the stomach. This establishes a better pH environment for the activity of the pancreatic enzymes.
Among these enzymes or their precursors are found trypsinogen, chymotrypsinogen and procarboxypeptidases which are activated in the small intestine. The mechanism for this is the activation of trypsin which is cleaved from trypsinogen by the intestinal enzyme enterokinase. This in turn activates its own and other enzyme precursors. Also in the exocrine pancreatic secretions is alpha amylase which hydrolyzes starches, lipases which act on triglycerides and phospholipids and other enzymes such as ribonuclease, elastase and collagenase.
What is the basal electrical rhythm displayed by gastrointestinal smooth muscle?
For peristalsis and other contractile events to occur, signals must be transmitted in a co-ordinated fashion between smooth muscle cells in the gut wall. Describe how the intrinsic properties of smooth muscle cells and the enteric nervous system promote such transmission.
The GI smooth muscle undergoes a rhythmic depolarization which is myogenic in origin. Slow waves can be initiated in neighbouring regions by passive current spread through gap junctions, so that the waves are conducted along and around the stomach wall. The ionic basis for the slow wave has been attributed to permeability changes associated with an influx of calcium ions followed by an
efflux of potassium ions. The slow waves of the stomach are larger than those int eh small intestine and may have small spikes superimposed onto them which initiate contractions. Also the slow wave itself may reach a potential at which contraction is initiated, the amplitude of the contraction is then dependent on the degree and the time for which threshold is exceeded. Vagal stimulation of cholinergic nerves or gastrin released from the distal stomach mucosa depolarizes the smooth muscle cells to cause these contractions. Interstitial cells of Cajal act as pacemakers to drive the electrical activity and this is propagated to adjacent smooth muscle cells. There is a general decrease in the frequency of contractions along its length due to the failure of distal portions of intestine to follow the high frequency and thus the taking over by new pacemaker regions which operate at a slower rate.
How are lipids absorbed across the gastrointestinal mucosa?
Lipids are not water soluble making their absorption a complicated issue. First of all, the large lipid droplets must be emulsified to smaller lipid droplets and these then stabilized to prevent coalescence. This is brought about by the shearing force provided by the gastric and intestinal motility and the presence of stabilizers including bile salts. Fat digestion is initiated by lingual lipase and gastric lipase liberating short and medium length fatty acids. The emulsification process provides an enormous surface area. Trypsin then converts procolipase to colipase in the lumen and this binds to pancreatic lipase converting TAGs to free fatty acids and 2-MAGs. These together with bile salts, lecithin, cholesterol and fat-soluble vitamins form micelles. These aggregations have hydrophobic interiors and hydrophilic exteriors to allow them to move through the aqueous environment. They diffuse to the luminal cellular membrane and MAGs leave the micelles and diffuse passively across the luminal membrane while free fatty acids are absorbed both by passive diffusion and an uptake mechanism. The micelles themselves do not cross the membrane. Chylomicrons are then synthesized inside the cell near the Golgi apparatus with the free fatty acids being reesterified to TAGs. The chylomicrons are then released by exocytosis across the basolateral membrane and are removed by the lymphatic system, entering the systemic circulation via the thoracic duct.
Write a note on the hormone gastrin.
Gastrin is itself a hormone secreted by gastrin cells, also called G cells. These cells are located in the pyloric glands in the distal end of the stomach. Gastrin is a large polypeptide secreted in two forms: a large form called G-34, which contains 34 amino acids , and a smaller form, G-17, which contains 17 amino acids . Although both of these are important, the smaller is more abundant.
When meats or other protein-containing foods reach the antral end of the stomach, some of the proteins from these foods have a special stimulatory effect on the gastrin cells in the pyloric glands to cause release of gastrin into the digestive juices of the stomach. The vigorous mixing of the gastric juices transports the gastrin rapidly to the ECL cells in the body of the stomach, causing release of histamine directly into the deep oxyntic glands. The histamine then
acts quickly to stimulate gastric hydrochloric acid secretion. Gastrin can also directly act on the oxyntic cells by triggering an increase in intracellular calcium concentration. This then activates the hydrogen potassium ATPase. Gastrin secretion can also be triggered by caffeine and wine in the stomach and by nervous activity in extrinsic pathways during the cephalic stage of secretion as well as local distension.
By what mechanisms does the stomach protect itself against autodigestion by H+ & pepsIn.
The first method by which the stomach does this is to operate a negative feedback system where low luminal gastric pH inhibits the release of gastrin from
G cells from the distal region. Thus the most significant stimulus of secretion via gastrin acting on ECL cells is closed off. Also the pits, the glands and the remaining mucosa of the stomach not involved in other secretions contain large numbers of mucus-secreting cells. These produce a bicarbonate rich secretion which gives an alkaline layer of protection for the stomach’s inner lining against the acidic lumen. The mucus traps the bicarbonate ions secreted by these cells and thus shields the stomach from the digestive effects of acid and pepsin. The hormones secretin, somatostatin and GIP inhibit the release of Gastrin.
What would be the consequences of removal of the terminal ileum?
The two main absorbed molecules in the terminal ileum are vitamin B12 and Bile salts. If the terminal ileum is removed then the absorption of both will be greatly diminished. In the case of the former, reduced Vitamin B12 concentration lead to maturation failure of RBCs in the bone marrow and if untreated pernicious anemia. In the case of the later, bile salts are essential for digestion and absorption of fats and also fat soluble vitamins. Should this process be greatly impeded as one would predict, severe nutritional defiencies would result.
How does the action of sympathetic and parasympathetic nerves affect GIT smooth muscle function?
What is the effect of the sympathetic nerves on gastrointestinal motility?
What is peristalsis? Show, with the aid of a diagram, how the enteric nerves regulate this function.
What is the primary mixing movement seen in the small intestine? What are the principal neural regulators of this event?
The three main mixing movements seen in the small intestine are segmentation, pendular contractions and peristalsis. Segmentation is the alternate contraction and relaxation of complete segments of the small intestine and reflects the activity of the circular muscle. The contractile band does not progress but the pattern has some propulsive movement because of decreased frequency of segmentation from duodenum to ileum. Pendular movements involve contraction of longitudinal muscle. This moves the wall of the intestine over the contents of the lumen and so serves to mix and propel the chime through the small intestine.
Peristalsis is the main propulsive force in the small intestine. These are moving,
ring like contractions preceded by a more distal region of relaxation as they move towards the colon. They can be elicited by luminal distension. During fasting however, a different pattern of motility is present, the interdigestive motor cycle.
This involves a short period of intense activity followed by a period of inactivity and then a period of irregular contractions. These bursts have the effect of emptying out the residual contents of the stomach, duodenum and small intestine.
This motility is dependent on myogenic, neural and hormonal mechanisms. The rhythmic depolarizations of the slow waves lead to smooth muscle contraction.
Both intrinsic and extrinsic nerves can regulate the motility pattern. Stimulation of the post-ganglionic cholinergic enteric nerves increases the amplitude of contractions, while stimulation of the sympathetic or non-adrenergic inhibitory nerves inhibits contractions. The neural regulation is mostly intrinsic however though extrinsic nerves play a role in reflexes. CCK is important in stimulating motility.
The myenteric plexus of the GIT is mostly responsible for control of motility.
Because the myenteric plexus extends all the way along the intestinal wall and because it lies between the longitudinal and circular layers of intestinal smooth muscle, it is concerned mainly with controlling muscle activity along the length of the gut. When this plexus is stimulated, its principal effects are (1) increased tonic contraction, or "tone," of the gut wall, (2) increased intensity of the rhythmical contractions, (3) slightly increased rate of the rhythm of contraction, and (4) increased velocity of conduction of excitatory waves along the gut wall, causing more rapid movement of the gut peristaltic waves.
The effects then of parasympathetic innervation is to enhance while sympathetic innervation inhibits this intensity.
Sympathetic innervation does this in two ways:
(1) to a slight extent by direct effect of secreted norepinephrine to inhibit intestinal tract smooth muscle (except the mucosal muscle, which it excites) and (2) to a major extent by an inhibitory effect of norepinephrine on the neurons of the entire enteric nervous system . Stimulation of the parasympathetic nerves causes general increase in activity of the entire enteric nervous system. This in turn enhances activity of most gastrointestinal functions.
Write a note on the role of the hormone secretin.
Secretin contains 27 amino acids and the full molecule is required for activity. It inhibits gastric acid secretion in a non-competitive way. It is produced by S cells found in the mucosa lining the duodenum and jejunum and is released into the blood in response to increased acidity and to the presence of fatty acids in the upper small intestine, either via a direct effect on the cells or indirectly through local nerve reflexes. It is carried in the blood and stimulates the duct cells in the pancreas and liver via cAMP to produce increased volumes of bicarbonate rich secretions. This effect is potentiated by CCK and the neurotransmitter ACh. In the stomach, secretin acts by direct action on oxyntic cells and by inhibition of gastrin release. Like CCK, it also stimulates the growth of acinar cells in the pancreas.
Describe the mechanism of hydrogen ion secretion by oxyntic (parietal) cells. How is this mechanism regulated?
Using a diagram of an oxyntic cell indicate the mechanism of [H+] secretion. How is acid secretion inhibited?
HCl is secreted by oxyntic cells. At rest these contain numerous vesicles rich in hydrogen potassium ATPases and lie adjacent to canaliculi which open into the lumen of the gastric glands. Then the cells are stimulated to secrete acid, the vesicles fuse with the canliculi and at the same time there is an increase in membrane permeability to potassium and chloride. The movement of potassium into the cell activates the hydrogen potassium ATPase so that a one for one exchange occurs and there is a net secretion of HCl. The water accompanying the HCl is driven out of the cell by the efflux of ions. Gastric secretion is stimulated by histamine, ACh and gastrin. Histamine acts in a paracrine fashion on H2 receptors of oxyntic cells and stimulates adenylyl cyclase via the G protein and by elevating the cAMP thus activating the ATPase. ACh raises the levels of calcium ions as does gastrin and all three act synergistically to stimulate greater acid secretion. Somatostatin on the other hand can prevent the release of histamine in response to gastrin or ACh and also directly inhibit the parietal cell.
The physiological stimulus for acid secretion is food. Another method of control is to operate a negative feedback system where low luminal gastric pH inhibits the release of gastrin from G cells from the distal region. Thus the most significant stimulus of secretion via gastrin acting on ECL cells is closed off.
What are the primary components of bile? List the digestive function of one of these components.
What is the fate of bile acids secreted into the small intestine?
Bile consists primarily of bile salts and bicarbonate ions. It serves as an excretory route for bile pigments, cholesterol, steroids, heavy metals and a variety of drugs.
The bile salts are important for their role in the digestion and absorption of fats and of fat soluble vitamins because of their emulsifying action. Free fatty acids and 2-MAGs with bile salts, lecithin, cholesterol and fat-soluble vitamins form micelles. These aggregations have hydrophobic interiors and hydrophilic exteriors to allow them to move through the aqueous environment. They diffuse to the luminal cellular membrane and MAGs leave the micelles and diffuse passively across the luminal membrane while free fatty acids are absorbed both by passive diffusion and an uptake mechanism. The micelles themselves do not cross the membrane but instead are reabsorbed throughout the small intestine by passive diffusion and sodium dependent secondary active transport. Bile acids are synthesized in the liver as cholic and deoxycholic acids and are conjugated with taurine or glycine to form bile salts. More than 90% of the bile acids secreted into the small intestine are reabsorbed and returned to the liver in the portal circulation mostly bound to plasma proteins in what is called the enterohepatic circulation.
What is the role of the interstitial cells of Cajal in GIT motility?
Most gastrointestinal contractions occur rhythmically, and this rhythm is determined mainly by the frequency of so-called "slow waves" of smooth muscle membrane potential. These waves are not action potentials. Instead, they are slow, undulating changes in the resting membrane potential. Their intensity usually varies between 5 and 15 millivolts, and their frequency ranges in different parts of the human gastrointestinal tract from 3 to 12 per minute: about 3 in the body of the stomach, as much as 12 in the duodenum, and about 8 or 9 in the terminal ileum. Therefore, the rhythm of contraction of the body of the stomach usually is about 3 per minute, of the duodenum about 12 per minute, and of the ileum 8 to 9 per minute. The precise cause of the slow waves is not completely understood, although they appear to be caused by complex interactions among the smooth muscle cells and specialized cells, called the interstitial cells of Cajal , that are believed to act as electrical pacemakers for smooth muscle cells. These interstitial cells form a network with each other and are interposed between the smooth muscle layers, with synaptic-like contacts to smooth muscle cells. The interstitial cells of Cajal undergo cyclic changes in membrane potential due to unique ion channels that periodically open and produce inward (pacemaker) currents that may generate slow wave activity. The slow waves usually do not by themselves cause muscle contraction in most parts of the gastrointestinal tract, except perhaps in the stomach . Instead, they mainly excite the appearance of intermittent spike potentials, and the spike potentials in turn actually excite the muscle contraction. The spike potentials are true action potentials. They occur automatically when the resting membrane potential of the gastrointestinal smooth muscle becomes more positive than about -40 millivolts (the normal resting membrane potential in the smooth muscle fibers of the gut is between -50 and -
60 millivolts). Thus each time the peaks of the slow waves temporarily become more positive than -40 millivolts, spike potentials appear on these peaks. The higher the slow wave potential rises, the greater the frequency of the spike potentials, usually ranging between 1 and 10 spikes per second. The spike potentials last 10 to 40 times as long in gastrointestinal muscle as the action potentials in large nerve fibers, each gastrointestinal spike lasting as long as 10 to 20 milliseconds. Another important difference between the action potentials of the gastrointestinal smooth muscle and those of nerve fibers is the manner in which they are generated. In nerve fibers, the action potentials are caused almost entirely by rapid entry of sodium ions through sodium channels to the interior of the fibers. In gastrointestinal smooth muscle fibers, the channels responsible for the action potentials are somewhat different; they allow especially large numbers of calcium ions to enter along with smaller numbers of sodium ions and therefore are called calcium-sodium channels . These channels are much slower to open and close than are the rapid sodium channels of large nerve fibers. The slowness of opening and closing of the calcium-sodium channels accounts for the long duration of the action potentials. Also, the movement of large amounts of calcium ions to the interior of the muscle fiber during the action potential plays a special role in causing the intestinal muscle fibers to contract, as we discuss shortly.
With the aid of a diagram show the principal mechanisms for nutrient absorption in the early proximal tubule.
Compare and contrast water absorption by the ileum and colon.
Indicate the location of absorbing and secreting cells on the intestinal villus.
Why is a sodium/glucose solution useful in rehydration following cholera infection?
Endocrinology
Write an essay on thyroid hormone.
Using one example explain the mechanism of negative feedback in the control of hormone release.