Extended Fin & Mathcad Intro II (w jpegs)
advertisement

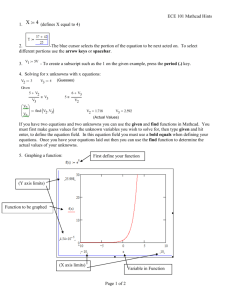
Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 1 Grading Sheet ~~~~~~~~~~~~~~ MIME 3470—Thermal Science Laboratory ~~~~~~~~~~~~~~ Laboratory 2 EXTENDED FIN / MATHCAD TUTORIAL 2 Student’s Name / Section № № OF DEVIATIONS (Minus 2 points per deviation) THE DELIVERABLE OF THIS LAB IS AN EXACT DUPLICATION OF PAGES 6 THROUGH 10. TWO (2) POINTS WILL BE DEDUCTED FOR EACH DEVIATION FROM AN EXACT DUPLICATION. A *.DOC FILE WITH CORRECT MARGINS, HEADER, ETC. CAN BE DOWNLOADED FROM THE COURSE WEB SITE WHERE THE *.PDF FILE WITH THE FULL REPORT IS ALSO AVAILABLE. COMMENTS d GRADER— TOTAL (100 PTS Max) Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 MIME 3470—Thermal Science Laboratory ~~~~~~~~~~~~~~ Laboratory №. 2 Experimental, Analytical, & Numerical Solutions for an EXTENDED FIN & MATHCAD TUTORIAL 2 ~~~~~~~~~~~~~~ PREPARED BY: STUDENT’S NAME SECTION: № ~~~~~~~~~~~~~~ Originally, this experiment was performed around the 10th week. The lab write up took at least twice as much time as a normal write up and there was much confusion as to what was required of the students. It was too aggressive a lab for a mere two-hour lab course. Thus, the lab was turned basically into a Mathcad tutorial for the 2nd week and then actual data will be taken in the 10th week. OBJECTIVE—Here the intention is to 1. Study the basic principles of conduction heat transfer in association with free convection cooling, 2. Demonstrate the utility of spike fins for heat transfer enhancement, 3. Illustrate the use of finite difference solvers in heat conduction, and 4. Further the student’s introduction to Mathcad. THEORY—The laws that describe heat transfer in a fin are Fourier’s Law of Heat Conduction and Newton’s Law of Cooling. Both are based on observation of physical phenomena and are constitutive relations. Fourier’s Law is based upon the fact that the conductive heat, Qx, is directly proportional to the negative of the temperature gradient. For one-dimensional heat conduction this is T Qx , (1) x where Qx heat flowing in the positive x direction, T absolute temperature at x, K. The negative sign is used because heat flows from higher to lower temperatures. For heat flow in the +x direction, the term dT/dx is negative. So for the inputted energy to be a positive value, the negative sign is used. Besides the temperature gradient, it should be apparent that the amount of heat let through is directly proportional to the cross sectional area normal to the direction of heat flow and will differ from one type of material to another. Introducing the thermal conductivity as the constant of proportionality representing the material, Fourier’s law is then expressed as T Qx kAXS , (2) x where AXS cross sectional area of fin, m2, k thermal conductivity of the fin material, W/m∙K. Heat passes out of an object and into a fin by conduction; but, the passage of that heat to the ambient air (or any other fluid) is defined by Newton’s law of cooling. This is not a law (but a constitutive relation defining the heat transfer coefficient, h) and is not about cooling, per se; however, the name has held. This relation is expressed by (3) Qs hAs Ts Tamb , where s subscript indicating the surface as opposed to the cross section of the fin, As surface area of fin having a perimeter of length P, m2, h heat transfer coefficient, W/m2∙K, Tamb ambient temperature of surrounding fluid, K. Analytical Solution—Consider the rod (spine) attached to a surface maintained at a constant temperature shown in Figure 1. Page 2 L T Tamb T Tb Cross Sectional dx x Area, AXS Figure 1—Fin attached to a surface of constant base temperature, Tb For the general, differential element shown in Figure 2, the steady-state energy balance can be written where all the heat in from the left-hand side of the element equals that passing out to the right-hand side plus the heat passed to the fluid via Newton cooling. In equation form this is kAXS dT dx x T Tamb Heat conducted out by convection Heat conducted out by conduction at x + x Heat conducted in by conduction at x x x c.v. Figure 2—Heat flow through a small element kAXS dT dx h As T Tamb x x Px dT dT dx x x dx x hP T T amb kAXS x m2 Taking the limit as x 0 yields d 2T dx 2 2 m T Tamb . (4) hP kA XS Note that the relation uses m2. Forgetting this has caused most engineers grief at one time or another. Two types of boundary conditions will be considered in this lab—these are: 1) Case A, Infinite Fin—The fin is infinitely long such that there is no tip to consider. If there were a tip at infinity, it would never see the heat introduced by the base in this century. Thus, at infinity, the condition always exists that T = Tamb. With T = Tamb at the tip, no difference in temperature exists to enter into Newton’s relation, and thus, no heat transfer to the ambient occurs at infinity. The above differential equation is more easily solved with a change in variable. Use = T Tamb. Then d2/dx2 = d2T/dx2 and then Equation 4 becomes d 2 (5) m2 0 . dx2 Assuming a solution of the form erx and substituting into the differential equation yields two roots r 2erx m2erx 0 r 2 m2 r m, m . So, the general solution is Ae mx Be mx . Apply the boundary conditions at x = , T = Tamb = finite, thus B = 0; at x = 0, T = Tb, thus A = Tb. Thus, the solution is T Tamb Tbemx . (6) Note: Tb must be an absolute temperature. 2) Case B, Tip Convection—Here there is convective (Newton’s law) heat transfer at the tip as well as the sides. Separation of variables is used to obtain a solution. The only difference between an undergraduate heat transfer course and a graduate conduction Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 course is the math. The undergraduate is simply told what relation is the solution to a certain set of boundary conditions. In a graduate course, the student gets to see how the relations are derived. Such derivations are not difficult once the mathematical underpinnings are taught. The solution to this case is h sinhmL x coshmL x mk (7) T Tamb h sinhmL coshmL mk T = Tb= T0 x i 1 i i 1 Cell Cell Half Half Cell Cell x N 1 N Cross Sectional Area, A XS Figure 3— Discretetized fin problem Numerical Solution—An alternate way of solving Equation 4 is to use a differenced form of the equation and solve for the temperature distribution, usually, iteratively. In such a procedure, the spatial domain (x distance along the fin) is discretetized into a number of segments or cells as shown in Figure 3. Heat flows into and out of these cells just as with the smaller differential element of Figure 2. The temperature throughout each cell is assumed to be constant—spatially lumped temperature. The numerical solution for the cell cannot grasp the concept that the segment has some x dimension—it only sees the cell as having one uniform temperature. Thus, it is convenient for the engineer to consider the entire cell as a point or node having the same temperature as the cell. Unless otherwise prompted, this node is usually positioned at the center of the cell. For this solution, the nodes are equally spaced with nodes at the tip and base. The objective of the numerical solution is to determine the temperature at each node, Ti. i Ein cond c.v. Eout cond Ti 1 2Ti Ti 1 x 2 hP Ti T . k rod AXS The solution procedures below require a single coefficient for each Tn. As Ti exists on the right-hand side of the equation, further rearrangement is needed to yield 2 1 hP 1 hP Ti 1 Ti T (13) Ti 1 2 2 krod AXS AXS x x 2 k x rod c a a b The terms a, b, and c are constants composed of dimensions and physical properties that do not vary with temperature. Now, consider the boundary conditions; i.e., the base and the tip. The equal spacing of nodes causes the cells at the base and tip to be half as long as the midbody cells. The temperature at the base node (i = 0) is already known, Ti–1 = T0 = Tb. So, for the first FULL cell (i = 1), the Equation 13 becomes x / 2 Ein cond iN Eout conv Eout conv Figure 5—Half cell at tip 2 hP 1 hP 1 .(14) T Tb Ti 1 Ti 2 k rod A XS k rod A XS x 2 x 2 x c a a i 1 b For the half cell at the tip, energy flows in by conduction. However, this energy flows out by convection from both the sides and the tip (see Figure 5). The differenced energy balance for this is k rod AXS TN TN 1 x h AXS P TN T . x 2 Again, grouping similar Tn terms to have a single coefficient for each yields Eout conv Figure 4—Heat flow through a cell With a knowledge of differencing, one could directly transform Equation 4 onto the new format. As the student may be new to this concept, the more basic concept of the energy balance will be again used. For the cell shown in Figure 4 having Node i at its center, the energy conducted in FROM Node (Cell) (i – 1) is: Ein cond krod AXS dT . (8) dx In differenced form, this differential equation is Ein cond k rod AXS T k rod AXS Ti Ti 1 . (9) x x Must be ( ). i.e., NOT Ti 1 Ti Similarly, energy conducted TO Node (i + 1) is: Eout cond krod AXS Ti 1 Ti . (10) x For uniform cell temperature and perimeter P, the lateral convection across the cell’s side area, As (=Px), is Eout conv hAs Ti T . (11) At steady state, the energy in equals that out; thus, one can write Ein cond Eout cond Eout conv Ti Ti 1 T T krod AXS i 1 i hPxTi T (12) x x Rearranging this gives the final form of the differenced equation krod AXS Page 3 k A x x k A TN 1 rod XS TN rod XS h AXS P T h AXS P . x x 2 2 d f e (15) What has been described by these differenced equations is a system of N linear equations with N unknown temperatures. Such can be solved using either of two methods: Solution to Simultaneous Equations Using Matrices—With this procedure the student should be familiar; but, a brief review is presented. Given a system of N linear equations, each of the form A1x1 A2 x2 AN xN B , such equations can be arranged in matrix form as A1, 2 A1, N x1 B1 A1,1 A2,1 A2, 2 A2, N x2 B2 . AN ,1 AN , 2 AN , N xN BN Α X B Substituting Equations 13, 14, and 15, into Equation 16 gives (16) Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 0 0 0 0 T1 c Tba b a 0 0 a b a 0 0 0 0 0 T2 c 0 a b a 0 0 0 0 T3 c 0 0 0 0 T4 c . 0 0 a b 0 0 0 0 b a 0 0 T8 c 0 0 0 0 a b a 0 T9 c 0 0 0 0 0 a b a T10 c 0 0 d e T11 f 0 0 0 0 Α B T (17) NN N 1 A 1 N N BN 1 Identity Matrix, I I N N XN 1 XN 1 A 1 N N BN 1 N 1 Equation 14, for Node 1, becomes, c Tb 0 a T2a , T1 b New (21) Value (18) NN (20) New Value Use Temperatures From Previous Iteration To properly use the inverse matrix, this simple relation must be maintained. The intent is to multiply both sides of the matrix equation by the inverse matrix of A, i.e., A1. The only position the A1can be placed and maintain the relation of Equation 18 is at the beginning of each side of the equation; i.e., 1 c Ti 1a Ti 1a , b Ti To solve for T, one cannot simply divide B by A as is done in simple algebra. Instead the inverse of Matrix A must be used. The reader will remember that there is a simple relation for the indices of A, T, and B. This is AA X Page 4 (19) N 1 This is the solution to X—or in the case of the fin, T. Solution Using Successive Iterations—Instead of a matrix solution, one can assume that all of the nodes of the rod are initially at room temperature and the base node is suddenly brought up to its steady state value, Tb. Then, in an iterative manner the nodes are heated as energy flows from the base node to the next, and the next,(refer to plots at the bottom of Page 9). This is time consuming but instructive. It is not the same as the actual problem as the base node is, in reality, slowly brought up to its steady-state temperature while simultaneously passing on energy to other nodes. Even though there is no real time increment between iterations, a similar process could be performed using time steps. When real time steps are used, some algorithms become unstable instead of converging to a solution. This is because the time change (step) is too big for the spatial increment, x. To obtain convergence, the time step is reduced—this process is called relaxation. An alternate form of relaxation is when a calculation tells one that the temperature at a node should be changed to some value in the next iteration but the programmer only programs in a percentage of the specified change. So the reader asks: Why use this method when the simpler matrix method provides an answer? The answer is that this lab course is teaching techniques. That the simultaneous equations are all linear is a fortuitous happening. Usually, one or more of the simultaneous equations are not linear and the matrix method is inapplicable. This method requires Equations 13, 14, and 15 to be rearranged to express the unknown temperature of any node, Ti, in terms of the temperatures of the adjacent nodes. Equation 13, for a general midbody node, becomes, Use Temperatures From Previous Iteration and, Equation 15, for the tip, becomes f TN 1d TN e New Value . (22) Use Temperatures From Previous Iteration With the base node at temperature Tb and after initializing all of the unknown temperature nodes (1N) to the ambient temperature, Tamb, the algorithm is performed by sweeping from Node 1 to Node N and at each node applying the appropriate equation. Thus, for the first sweep, only the temperature of Node 1 can be altered. With successive sweeps (iterations), the temperatures further out along the fin begin to change. The solution is reached when the change in temperature of all nodes is less than a user specified tolerance. A visual inspection of the change in temperature value at each node could be performed in stead of using a tolerance, per se. With no change in temperature between iterations, this solution is the steady-state temperature distribution of the fin as steady-state means no change. Empirical, Natural Convection Correlations—Here are summarized appropriate empirical correlations that have been developed for common immersed (external flow) geometries. The correlations are suitable for most engineering calculations and are usually of the form hL NuL CRa nL . (23) k where, h average heat transfer coefficient, W/m2∙K. For turbulent flows, this is independent of L, C Coefficient which is a function of Ra L (Fig. 6), L characteristic length of the geometry, m, k thermal conductivity of fluid, W/m∙K, n = 1/4 or 1/3 for laminar or turbulent flow, respecttively (function of Ra L , see Figure 6) Ra L Rayleigh number gTs T L3 Grashof number = GrL Pr GrL (24) gTs T L3 Prandtl number C p = k acceleration of gravity, m/s2, 1/Tf , K1, Ts Tamb / 2 , K, = Pr g Tf Ts surface temperature of fin, K, thermal diffusivity of fin, m2/s, and kinematic viscosity (aka viscous diffusivity) of fluid, m2/s. Note: all properties are evaluated at the film temperature, Tf, Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Note also: in the real fin of this lab, the surface temperature along the fin, Ts, is not constant (not isothermal). However, glancing ahead to Figure 8, this temperature varies little in terms of absolute temperature. Thus, for this lab, an average value of surface temperature will be used in computing film temperature, Tf. Page 5 base of the rod will be at some lower (but constant) temperature. DO NOT let the water in the container boil away. Once steam can be observed coming out of the vent pipe, take temperature readings generated by the eleven thermocouples mounted in the fin. Wait, say, ten minutes and measure the temperatures again. Wait another five or ten minutes and again measure the temperatures. When the temperatures for each thermocouple stop changing, steady state heat transfer has been reached. At steady state, the heat loss from the rod will exactly equal the heat added through its base and the temperature at any given point along the rod will remain unchanged with time. For the calculations, use only the last, steady-state values recorded. The equations developed above are for free or natural convection, students should avoid excessive talking or moving around as this induces forced air currents which will increase the convection from the fin and lower the temperatures being measured. To demonstrate this, choose one thermocouple and simply blow air over it. It will be observed that the temperature readout from that thermocouple immediately drops. Note also: there are other air currents in the lab which increase the heat flow from the fin—this will be considered. Tb 360 Figure 6—Free convection Nusselt number for vertical plate Natural Convection along a Vertical Plate—Equations of the form given by Equation 23 have been developed for the vertical plate and are plotted in Figure 6. The coefficient C and the exponent n depend on the Rayleigh number range; for Rayleigh numbers less than 104, the Nusselt number should be obtained directly from the figure. More recently, the following correlation has been recommended that may be applied over the entire range of RaL 2 0.387Ra1L/ 6 (25) NuL 0.825 . 8 / 27 1 0.492/Pr 9 / 16 The foregoing results may be applied for constant heat flux (e.g., a heating filament), as well as for constant surface temperature (isothermal). They maybe also applied to vertical cylinders of height L, if the boundary layer thickness, , is much less than the cylinder diameter, D. This condition is known to be satisfied when D / L ~ 35 / Gr1 / 4 . L Again, the real fin is not isothermal; but, an approximate procedure for determining this variation is to use Nu L correlations obtained for the isothermal plate but Ra L of Equation 24 (and thus Nu L ) should be defined in terms of the average surface temperature along the fin. Figure 7—Extended Fin Experimental Set Up (Photo: Greg Hill, BSME ‘03) EXPERIMENTAL PROCEDURE—A rod penetrates the top of an aluminum container as shown in Figure 7. The container is partially filled with water and heated using an electric heating element. When the water boils and steam can be seen coming out of the vent pipe, it can be assumed that the portion of the rod inside the container, as well as the inside surfaces of the container, are at the saturation temperature of water. The outside surface of the container and the Tamb L 300 240 Texp 180 i 120 60 0 0 0.1 0.2 0.3 0.4 x 0.5 0.6 0.7 i Figure 8— Experimentally measured, absolute temperatures, Texp, along the length of the fin, L. These are bounded by the base temperature, Tb, and the ambient temperature, Tamb. Note that the change in ABSOLUTE temperatures is not great. This is the basis of assuming an isothermal rod. CALCULATION PROCEDURE 1. Experimental—Graph the experimental data and fit a curve to that data. This curve will be used to compare other curves (see below). 2. Analytical—Using the base temperature from the steady-state data, plot curves for Case A and Case B along with the experimental data. It should be obvious from Item 1 that the fin is not isothermal. Yet, if the experimental temperature distribution is plotted using absolute temperatures as shown in Figure 8, the change in temperature over the length of the rod is small. Thus, this experiment assumes an isothermal bar for its calculations. The example of Appendix B may be helpful in these calculations. 3. As the actual data will not reflect pure, natural convection, but will also reflect other air currents near the experiment, the temperatures of the experimental data will be lower than those predicted by Cases A and B. These two cases used an average heat transfer coefficient developed Equation 26 ( h NuLkair / L ).Manually change the average heat transfer coefficient until the curve for the more realistic Case B lies atop the curve for the experimental data. 4. Numerical—Use this new value of heat transfer coefficient to perform numerical solutions for the temperature distribution using the matrix method and method of successive iterations. Plot experimental, tip convection, matrix, and successive iteration temperatures on one graph. Explain any discrepancies in the results. NOTE: Thermocouple spacing does not match the equally spaced nodes used to develop the differenced equations. Do not try to match the thermocouple spacing; instead use 10 evenly spaced nodes. Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 ORDERED DATA, CALCULATIONS, AND RESULTS— Read “BEFORE STARTING this Mathcad tutorial” on Page 10. Page 6 Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 7 Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 8 Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 9 Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 10 BEFORE STARTING this Mathcad tutorial, note the following: 1. When using a Mathcad object within MS Word, occasionally, when one opens the object, all of their work from a previous session just disappears. Although part of one’s engineering education is to learn to fiddle with things which do not work as advertised, the student does not need this extra aggravation. Thus, it is recommended that the student prepare his/her Mathcad work using Mathcad as a stand-alone program. Then, paste that work into a Mathcad object when done. So one asks: Why even bother to use Mathcad objects? The answer is that the student is learning to produce seamless documents with text, equations, graphics, and other objects which will impress one’s boss and clients—and be easy to grade by the TA. 2. To present a multipage Mathcad calculation in an MS Word document (such as the 5 objects just above), note that the individual objects do not communicate with one another. Thus, the Mathcad programming shown in each object must be preceded by the appropriate programming. So, after one prepares his/her work using just Mathcad as discussed in Item 1, paste that work INTO a Mathcad object. Then for the second Mathcad page to be shown; copy the Mathcad object and paste it below the first one and then open the second object and scroll down until “the work of the second page” is properly aligned in the object’s window. Close the object and proceed to the next “page” if there is one. 3. Mathcad has a number of predefined variables and units; e.g., “g” is the acceleration of gravity and “A” is amperes. If the user defines A as, say, area, Mathcad will place a green squiggle under the A to warn the user that that the predefined variable is being redefined. This is good!; but, sometimes annoying. To turn off the squiggles, type [CTRL + SHIFT + r]. 4. Depending on the version of Mathcad, the units of a variable may be reported differently; e.g., m/s as to m s-1. Ignore differences. DISCUSSION OF RESULTS (For actual lab later in term) CONCLUSIONS (For actual lab later in term) Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 11 APPENDICES Appendix A—PHYSICAL PROPERTIES (@300K) Copper Density, Specific heat, Cp Thermal conductivity, k Thermal diffusivity, = k/ Cp Air 8933 kg/m3 385 J/kgK 401 W/mK 117 m2/s Appendix B—EXAMPLE PROBLEM—A glass-door firescreen, used to reduce exhilaration of room air through a chimney, has a height of 0.71m and a width of 1.02m and reaches a temperature of 232C. If the room temperature is 23C, estimate the convection heat rate from the fireplace to the room. Solution Known: Glass screen situated in fireplace opening. Find: Heat transfer by convection between screen and room air. Schmetic: Density, 1.1614 kg/m3 Specific heat, Cp Kinematic viscosity, Thermal conductivity, k Thermal diffusivity, = k/ Cp Prandtl Number 1.007J/kgK 15.89106 m2/s 26.3103 W/mK 22.5106 m2/s 0.707 and from [another relation] it follows that transition to turbulence occurs on the panel. The appropriate correlation is given by Equation 25. 0.387Ra 1L/ 6 Nu L 0.825 1 (0.492 / Pr) 9 / 16 and where h may be obtained from knowledge of the Rayleigh number. Using Equation 24, 9.8 m 1 232 23C 0.71m 3 s 400 K gTs T L3 Ra L m m 38.3 10 6 26.4 10 6 2 s s2 1.813 10 9 APPENDIX C—HISTORICAL SKETCHES Baron Jean Baptiste Joseph Fourier (1768-1830) This French Mathematician and Physicist, famous for his pioneer work on the representation of functions by trigonometric series, was born at Auxere, France on March 21, 1768. He was the son of a tailor who became a teacher of mathematics at age sixteen at the military school in Auxere. He later joined the faculty at the Ecole Normale at Paris in the year of its founding (1795) when he was twenty-seven. His teaching success soon led to the offer of the Chair of Analysis at the Ecole Polytechnique and in 1807, he was made a member of the Academy of Sciences. Nu L k L 147 33.8 10 3 q hATs T Analysis:—The rate of free convection heat transfer from the panel to the room is given by Newton’s law of cooling, q h As Ts T , 2 2 1/ 6 0.387 1.813 10 9 0.825 147 8 / 27 9 / 16 1 (0.492 / 0.690) Hence, h Assumptions: 1. Screen is at a uniform temperature Ts. 2. Room air is quiescent. Properties: From a properties table, Air (Tf = 400K): k = 33.810-3 W/mK = 26.410-6 m2/s = 38.310-6 m2/s Pr = 0.690 = (1/Tf) = 0.0025K –1 8 / 27 W 7.00 2 m K W mK 0.71m 7.00 W m2 K 1.02m 0.71m 232 23C 1060W Comments: 1. If h were computed from Equation 23, with C = 0.10 and n = 1/3, we would obtain h 5.8W / m 2 K and the heat transfer predicttion would be approximately 20% lower than the foregoing result. This difference is within the uncertainty normally associated with using such correlations. 2. Radiation heat transfer effects are often significant relative to free convection. Using Equation 1.6 and assuming = 1.0 for the glass surface and Tsur = 23C, the net rate of radiation heat transfer between the glass and the surroundings is 4 q As Ts4 Tsur W 232 2734 23 2734 11.02 0.71m 2 5.67 10 8 2 m K4 2355W Hence in this case radiation heat transfer exceeds free convection heat transfer by more than a factor of 2. Fourier's masterpiece was his mathematical theory of heat conduction stated in Theorie Analytique de la Chaleur (1822). As one of the most important books published in the 19th-century, it marked an epoch both in the history of pure and applied mathematics. In it, Fourier developed the theory of the series known by his name and applied it to the solution of boundary-value problems in partial differential equations. This work brought to a close a long controversy, and henceforth it was generally agreed that almost any function of a real variable can be represented by a series involving the sines and cosines of integral multiples of the variable. After a long and distinguished career, Fourier died in Paris on May 16, 1830 at age 62. http://www.me.utexas.edu/~me339/Bios/fourier.html See also http://wwwgap.dcs.st-and.ac.uk/~history/Mathematicians/Fourier.html Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Sir Isaac Newton (1643-1727) Re: what is the history behind Newton's law of cooling? Date: Sat Nov 4 23:02:29 2000 Posted By: Benjamin Monreal, Grad Student, Physics, MIT Area of science: Physics ID: 973198815.Ph Message: Re: [HM] Finite difference arrays -----Message d'origine----De : M. Robert Showalter <showalte@vms2.macc.wisc.edu> A : historia-matematica@chasque.apc.org Date : mercredi 27 janvier 1999 16:25 Objet : [HM] Finite difference arrays > ... I'm sure these finite difference arrays must have a history that goes back farther. It seems likely that finite differencing arrays for calculating polynomials must have been familiar objects for many mathematicians in times past. How might I trace the matter back farther? What resources might I use? Could someone please tell me more about the history of these finite difference arrays? Bonjour Anna, You're (sort of) in luck... Newton presented the Law of Cooling in a paper [published anonymously] entitled "Scala Graduum Caloris", published (in Latin of course!) in the Philosophical Transactions of the Royal Society of London, in 1701. I found an English translation of this paper online at http://web.bham.ac.uk/winterhs /Newton.htm (Isn't the Web amazing!) However, reading 300-year-old scientific papers is even harder than reading modern ones. I'll try to sum it up for you, which should make it easier to follow. Page 12 Anne Michel-Pajus (Annie.Michel-pajus@wanadoo.fr) Thu, 28 Jan 1999 07:38:34 -0000 Some indications (not exhaustive): Finite differences are related to the problem of interpolation, and construction of tables. http://artzia.com/History/Biogra phy/Newton/ Basically, Newton had to make up his own temperature scale— you http://astro.if.ufrgs.br/newton/ couldn't just buy a Celsius thermometer back in 1701—so the first part of the paper discusses how he did this. He thought up a bunch of "reference temperatures"—the temperature at which water boils, the temperature at which metals glow faintly, the temperature at which bismuth melts, etc. He measured the temperatures of these events by asking "How much does 10,000 units of volume of linseed oil expand at this temperature"? Imagine building a crude thermometer, filled with linseed oil instead of mercury. You raise the temperature of the thermometer, the oil expands and rises up the tube. Then, instead of writing numbers 0-100 on the tube and calling them "degrees Celcius", Newton put little marks on the thermometer and labeled them "lead melts here; water boils here; bismuth melts here" and so on. Newton then put his own numerical scale on the tube, running from 0 (ice) to 192 (red-hot coals), and called the units "degrees of heat". Then, for the cooling law itself, he heated a "pretty thick piece of iron red-hot". It was put in a cool place, and samples of the "temperature flags" were placed on it—a bit of bismuth, a bit of lead, a drop of water, etc. Newton observed how long it took before the bar cooled enough to solidify the lead, solidify the bismuth, to stop glowing, etc., and comparing these times to the marks on his imaginary linseed thermometer, he deduced that the rate of heat loss was linear with temperature; i.e. "The excess of degrees of heat [temperatures] of the iron... were in geometrical progression, when the times were in arithmetical progression." I don't know whether he'd invented enough calculus yet to turn the idea of "geometrical progression" into a differential equation, but that's how the experiment was done. Interesting stuff indeed! And it gives you an idea of some of the difficulties of doing science way back then... you had to invent your own temperature scales, know all sorts of chemistry, do all of the math by hand, etc. Newton was an alchemist, remember, so these sort of games with bismuth and tin and red-hot iron was right up his alley. Hope this helps! Thanks for asking! -Ben Monreal MadSci Network: Physics http://www.madsci.org/posts/archives/nov2000/973522810.Ph.r.html They are used by Indian and Arab mathematicians: among others, Brahmagupta, in 665 (Khanda Khadyaka), and Al-Biruni (X-XI, Qanun al-Mas'udi) for the calculation of sinus tables for astronomy. Then by Briggs for the tables of logarithms (Aritmetica logarithmica, 1624), with methods maybe found by Harriot (around 1610). A general formula is given by Gregory (letter to Collins 1670), but the main work is done by Newton (from 1675, letter to John Smith, then in Methodus differentialis, Letter to Oldenburg 1676, Principia...) Leibniz (in Historia and Origo... 1715) tells that finite differences are the source of his invention of Calculus. Bibliography: History of algorithms, Chabert et alii, Springer-Verlag, translation of Histoire d'Algorithmes, Belin. Best wishes from Versailles, Anne Michel-Pajus http://sunsite.utk.edu/math_archives/.http/hypermail/historia/jan99/0171.html History of heat transfer This doesn't pretend to be a complete history but I am slowly building up a few things that have caught my eye. Dividing heat transfer up into conduction, convection and radiation it is odd that each of these has a famous name attached to it and that the names in question are probably better known for something else. The understanding of conduction certainly started with Fourier, although he is probably better known for Fourier series. Modern understanding of radiation is certainly due to Planck who produced the theoretical law that gives the energy distribution with wavelength, from which the other laws can be proved. He is better known for inventing quantum mechanics (e.g. Planck's constant), which first appeared in his radiation theory. Much earlier Newton started the study of forced convection heat transfer with Newton's law of cooling. Again he is more famous for something else, though precisely what escapes me at the moment. Anyway, all I have at the moment in my history is Newton's original paper . http://web.bham.ac.uk/winterhs/History.htm Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 13 APPENDIX D—DATA SHEET FOR EXTENDED FIN EXPERIMENT Time/Date ____________________________ Lab Partners ____________________________ ____________________________ ____________________________ ____________________________ Data Rod Diameter Rod Length Distance from Base to 2nd Thermocouple Distance Between Thermocouples Distance from Last Thermocouple to Tip Rod Material Ambient Temperature . 3/8” . 23” . 3/4” . 2-1/2” . 3/4” . Copper . 72°F Sketch of Apparatus with Appropriate Dimensions Temperature Readings—When ALL the temperatures cease to change with time, steady-state has been achieved. Such will be the last (not necessarily 5th) column of numbers recorded. The steady-state readings are the values to be used in the calculations. Thermocouple Number Run 1 Run 2 Temperature Reading Run 3 Run 4 Run 5 1 173°F 177 176 174 2 169 172 171 168 3 157 163 163 162 4 130 135 135 132 5 122 129 129 130 6 112 117 118 117 7 105 111 114 113 8 100 105 107 108 9 96 101 104 104 10 94 99 102 102 11 92 96 98 99 Last Rev.: 23 JLY 08 Extended Fin / Mathcad Tutorial 2 : MIME 3470 Page 14