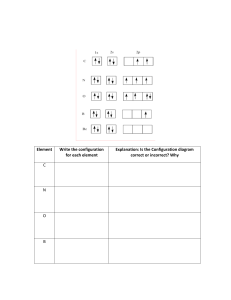
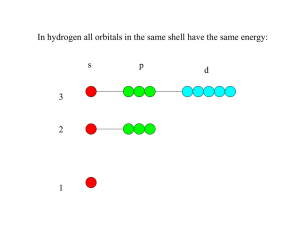
PPT 3
advertisement
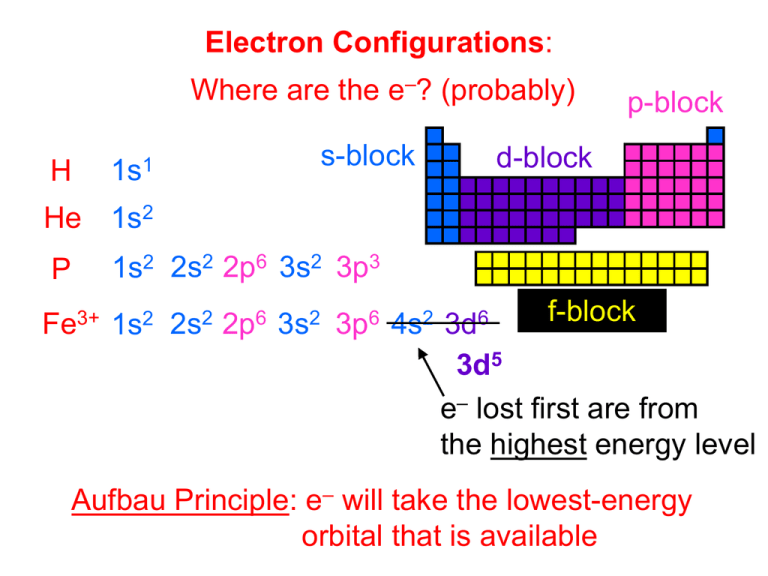
Electron Configurations: Where are the e–? (probably) H 1s1 s-block p-block d-block He 1s2 P 1s2 2s2 2p6 3s2 3p3 f-block Fe3+ 1s2 2s2 2p6 3s2 3p6 4s2 3d6 3d5 e– lost first are from the highest energy level Aufbau Principle: e– will take the lowest-energy orbital that is available Al3+ 1s2 2s2 2p6 3s2 3p1 O2– 1s2 2s2 2p64 F– 1s2 2s2 2p56 N3– 1s2 2s2 2p36 isoelectronic species: species having the same e– configuration “equal-energy” Hund’s Rule: each degenerate orbital must have one e– before any take a second Friedrich Hund (1896–1997) e.g., the three 3p orbitals are degenerate Orbital Diagrams …show spins of e– and which orbital each is in O 1s 2s 2p 3s 3p 1s 2s 2p 3s 3p P paired e– unpaired e– valence e–: the s and p e–s (ONLY!) in the outermost energy level Sections of Periodic Table to Know s-block p-block d-block f-block Shorthand Electron Configuration (S.E.C.) 1. Put symbol of noble gas that precedes element in brackets. 2. Continue writing e– config. from that point. S [Ne] 3s2 3p4 Co [Ar] 4s2 3d7 In a many-electron atom, each e– is attracted to the nucleus and repelled by the other e–. -- effective nuclear charge, Zeff: the net (+) charge attracting an e– (a measure of how tightly particular e–s are held) Z = atomic number Equation: S = # of e– BETWEEN nucleus and e– in question (NOT e– Zeff = Z – S in same subshell) -- Within a given electron shell, Fe s e–s have the greatest Zeff, f e–s the least. For Fe, the 3p e–s have Zeff = 26 – 12 = 14; the 3d e–s have Zeff = 26 – 18 = 8. 3d6 4s2 3p6 3s2 2p6 2s2 1s2 nuc. -- The (+) charge “felt” by the outer e– is always less than the nuclear charge. This effect, due to the core (or kernel) electrons, is called the... screening effect (or shielding effect). v.e– Li v.e– K tougher to remove easier to remove