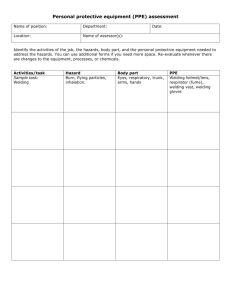
DraftReport2002(G22) - School of Mechanical Engineering
advertisement

Investigation of Hydrogen Assisted Cold Cracking in Low Alloy Steel Authors: Kar Keat Ch’ng Kin Mun Cheah DEPARTMENT OF MECHANICAL ENGINEERING Final Year Project Report for Partial Fulfillment for the Bachelor of Engineering (Mechanical / Mechatronic) October 2002 Abstract Hydrogen Assisted Cold Cracking (HACC) is a serious and common problem that can cause catastrophic failure at welded joints of steel structures. Therefore the investigation into the cause of HACC is extremely important and beneficial as it can provide more information on methods of prevention of HACC. The aim of this project is to examine the effects of different welding conditions on mild steel that could lead to segregation of alloying elements within the weld during solidification. Segregation is believed to contribute to localized higher carbon equivalent and hence increasing the susceptibility of the welded joint to HACC. This project progressed in three stages. In the initial stage, discussions were held to evaluate and decide on the appropriate type of welding process, material, weld joint configuration and welding parameters to be employed throughout the project. Welding was conducted in the Mechanical Engineering workshop with the aid of an automated welding machine and numerous welds were produced on mild steel plates. The second stage involved preparation of metallographic specimens. This involved cutting welded samples to produce transverse sections of the welds, which were subsequently mounted, polished and etched. Microscopic analysis was performed in the third stage, where the specimens were analysed using both optical and electron microscopes. The microstructures of the weld metal in each specimen were examined at different levels of magnification in search for the occurrences of segregation as well as the distinctive signs of HACC. II Declaration The work presented in this final year report has not been submitted, in full or in part, for another degree at this or any other institution. The contribution of others to the content of this report and all previously published material has been fully acknowledged. We give consent to this copy of our report, deposited in the university library, being available for loan and photocopying. ------------------Kar Keat Ch’ng ------------------Kin Mun Cheah III Acknowledgement The authors wish to thank and convey their appreciation to their supervisor, Mr. Ian Brown who has provided invaluable guidance and encouragement. His patience, suggestions and generosity in imparting his profound knowledge in metallurgy have been vital in the course of this project. Many thanks are going to Dr. Graham Powell for his help in metallographic preparation for the welded specimens in material laboratory. Thanks are due to Dr. Peter Self, senior electron microscopist from the Centre of Electron Microscopy and Microstructure Analysis (CEMMSA) for conducting training sessions on how to operate the electron microscope with integrated Energy Depressive X-ray Analyser (EDAX) and its software. Thanks are also extended to the helpful workshop technicians in the Holden Laboratory for their contribution in fabricating the equipment required in this project and especially to Ron Jager for his willingness to sacrifice his valuable time to demonstrate to us how to set up and operate the welding apparatus. The authors also would like to thank our friends who have contributed in one way or another in this project. IV Table of Contents Abstract ........................................................................................................................ II Declaration.................................................................................................................. III Acknowledgment ........................................................................................................ IV Table of Contents ......................................................................................................... V List of Figures ............................................................................................................. IX List of Tables ............................................................................................................ XII 1. Introduction ............................................................................................................ 1 2. Literature Review ................................................................................................. 3 2.1. Hydrogen Assisted Cold Cracking............................................................... 3 2.1.1. Factors Influencing HACC .............................................................. 3 2.1.1.1. Sources Of Hydrogen ................................................................. 3 2.1.1.1.1. Hydrogen From The Welding Consumable ................... 5 2.1.1.1.2. Hydrogen From The Atmosphere .................................. 6 2.1.1.1.3. Hydrogen From The Parent Steel .................................. 6 2.1.1.2. Stress (Applied or Residual) ...................................................... 6 2.1.1.3. Susceptibility Of Microstructure................................................ 8 2.1.1.3.1. Carbon Equivalent ......................................................... 8 2.1.1.3.2. Susceptibility Of Different Steel Types ....................... 11 V 2.1.2. 2.2. Important Characteristics Of HACC .............................................. 11 Solidification Of Fusion Weld ................................................................... 14 2.2.1. Microstructure Of Metals ............................................................... 14 2.2.2. Formation Of Solid Solution In Weld ............................................ 17 2.2.3. Microsegregation ........................................................................... 18 2.2.4. Macrosegregation ........................................................................... 19 3. Part 1 – Welding................................................................................................... 20 3.1. Welding Process......................................................................................... 20 Process Selection ........................................................................... 20 3.1.2. Fundamentals Of Process ............................................................... 21 3.2. 3.1.1. Material ...................................................................................................... 22 Grade AS 1018 Carbon Steel ......................................................... 22 3.2.2. API 5L X80 Steel ........................................................................... 23 3.2.3. Flux Cored Wire ............................................................................ 24 3.3. 3.2.1. Equipment .................................................................................................. 26 Welding Machine ........................................................................... 26 3.3.2. Carriage .......................................................................................... 29 3.3.3. Shielding Gas ................................................................................. 31 3.3.4. Welding Torch ............................................................................... 32 3.3.5. Base Support .................................................................................. 33 3.3.6. Personal Protective Equipment ...................................................... 34 3.4. 3.3.1. Welding Conditions ................................................................................... 34 Joint Geometry ............................................................................... 34 3.4.2. Electrode Polarity........................................................................... 35 3.5. 3.4.1. Welding Parameters ................................................................................... 35 3.5.1. Welding Current............................................................................. 35 3.5.2. Arc Voltage .................................................................................... 36 3.5.3. Electrode Extension ....................................................................... 36 VI 3.5.4. Travel Speed .................................................................................. 37 3.5.5. Electrode Angle ............................................................................. 38 3.5.6. Heat Input....................................................................................... 40 3.6. Welding Procedures ................................................................................... 40 3.7. Discussions ................................................................................................ 41 3.7.1. Test/Trial Run ................................................................................ 41 3.7.2. Lack Of Penetration ....................................................................... 42 3.7.3. Surface Porosity ............................................................................. 43 3.7.4. Reduction Of Joint Gap ................................................................. 43 3.7.5. Weldability..................................................................................... 44 3.7.6. Misalignment ................................................................................. 45 4. Part 2 – Metallographic Preparation ................................................................. 47 4.1. Cutting........................................................................................................ 47 4.2. Mounting .................................................................................................... 47 4.3. Grinding ..................................................................................................... 47 4.4. Polishing .................................................................................................... 48 4.5. Etching ....................................................................................................... 49 4.6. Discussions ................................................................................................ 51 5. Part 3 – Microscopy Analysis.............................................................................. 52 5.1. Optical Microscopy.................................................................................... 52 5.2. Scanning Electron Microscopy .................................................................. 52 5.3. Electron Probe Micro-Analysis (EPMA) ................................................... 53 6. Results And Discussions ...................................................................................... 56 6.1. Weld Microstructure .................................................................................. 56 6.2. Analysis Of Microsegregation And Macrosegregation ............................ 56 VII 7. Conclusions ........................................................................................................... 57 8. Appendix .............................................................................................................. 58 9. References ............................................................................................................. 59 VIII List of Figures Fig. 1 Different Stages Of Project In Order Of Progression .......................................... 2 Fig. 2 Solubility Of Hydrogen With Respect To Temperature Variation For A Typical Weld Metal..................................................................................................................... 4 Fig. 3 Diffusion rate of hydrogen through ferritic steel decreases as temperature falls. 5 Fig. 4 Residual stress distributions ................................................................................ 7 Fig. 5 Distribution of residual stress along a weld corresponding to the temperature change ............................................................................................................................ 8 Fig. 6 Comparison Of Tensile Strength Against Carbon Equivalent............................. 9 Fig. 7 Fisheye On The Fracture Surface ...................................................................... 13 Fig. 8 Growth of a Crystal ........................................................................................... 15 Fig. 9 The Dendritic Solidification Of A Metal ........................................................... 16 Fig. 10 A Body-Centred Cubic Structure .................................................................... 17 Fig. 11 Microsegregation Occurring At Crystal Boundaries ....................................... 19 Fig. 12 Gas shielded FCAW ........................................................................................ 22 Fig. 13 Welding Equipments ....................................................................................... 26 IX Fig. 14 PROMIG 500 ................................................................................................... 27 Fig. 15 Wire-Feeder Machine ...................................................................................... 28 Fig. 16 Main Function Panel ........................................................................................ 29 Fig. 17 Carriage ........................................................................................................... 30 Fig. 18 Carriage Speed Controller ............................................................................... 30 Fig. 19 Switch .............................................................................................................. 33 Fig. 20 Electrode Polarity ............................................................................................ 35 Fig. 21 Electrode Extension ......................................................................................... 37 Fig. 22. Formation of weld bead profiles ..................................................................... 38 Fig. 23 Travel And Work Angle .................................................................................. 39 Fig. 24 Welding Techniques ........................................................................................ 39 Fig. 25 Surface Porosity............................................................................................... 43 Fig. 26 Carbon Equivalent Value Based On The Nominal Composition Of Weld Metal With Respect To The Amount Of Dilution.................................................................. 45 Fig. 27 (I) Before Etching (Ii) After Etching ............................................................... 49 X Fig. 28 Mapped region in the weld .............................................................................. 53 Fig 29. Line scan within the weld metal with the composition of certain elements .... 54 XI List of Tables Table 1 - Typical Chemical Composition (%Wt) Of Grade AS 1018 Steel ................ 22 Table 2 - Mechanical Properties Of Grade AS 1018 Carbon Steel ............................. 22 Table 3 - Typical Chemical Composition (%Wt) Of Grade X80 Steel ....................... 23 Table 4 - Mechanical Properties Of Grade X80 Steel ................................................. 23 Table 5 – Composition Of The Flux Core Wire .......................................................... 24 Table 6 – Typical mechanical properties of weld metal .............................................. 24 Table 7 - Speed Of The Carriage For Different Speed Knob Number ....................... 31 Table 8 – Composition Of Shielding Gas Used ........................................................... 32 Table 9 – Summary Of Welding Conditions ............................................................... 42 Table 10 – Values Of Heat Input Corresponding To Welding Parameters ................. 42 XII 1. Introduction HACC of steel is a well characterized, well documented, and well-understood imperfection in welding. Enormous effort has been put forth to avoid the presence of HACC in welding; however, the efforts have not fully eliminated the occurrence of such failures. The aim of this project is to conduct an investigation on hydrogen assisted cold cracking in low alloy steel. Hydrogen assisted cold cracking is defined as cold cracking of weld due to the influence of hydrogen entrapment in the weld. Other names that refer to this phenomenon include hydrogen embrittlement, hydrogen cracking, hydrogen-induced cracking (HIC), delay cracking and cold cracking. Details of HACC will be discussed in the chapter two. Through out this report, the abbreviation HACC will be used as a representation of hydrogen assisted cold cracking. Since HACC itself is a broad and general topic, this project mainly concentrates on the correlation between segregation and HACC. In order to examine this correlation, welds were subjected to different modes of analysis. These welds are produced on low strength steel under numerous welding conditions, as part of the project requirement. Segregation is expected to occur on macroscopic and microscopic scale, known as macrosegregation and microsegregation respectively. These two types of segregation will influence HACC in different manners. This project progressed in three stages. In the initial stage, discussions were held to evaluate and decide on the appropriate type of welding process, material, weld joint configuration and welding parameters to be employed throughout the project. Welding was conducted in the Mechanical Engineering workshop with the aid of an automated welding machine and numerous welds were produced on mild steel plates. Details can be found in the third chapter of this report. 1 The second stage involved preparation of metallographic specimens. This involved cutting welded samples to produce transverse sections of the welds, which were subsequently mounted, polished and etched. Details of metallographic preparation are mentioned in chapter four. Microscopic analysis was performed in the third stage, where the specimens were analysed using both optical and electron microscopes. The microstructures of the weld metal in each specimen were examined at different levels of magnification in search for the occurrences of segregation as well as the distinctive signs of HACC. Results of the examinations are delivered in chapter five. The project flow is summarized and illustrated in Figure 1. Welding Process Metallographic Preparation Microstructure Analysis Fig. 1 Different Stages Of Project In Order Of Progression 2 2. Literature Review 2.1 Hydrogen Assisted Cold Cracking HACC is the result of embrittlement of ferritic steel, especially to welded steel due to the influence of hydrogen. HACC usually develops in the heat affected zone (HAZ) of the parent metal and weld metal. HAZ is the part of the parent material that is metallurgically affected by the heat that is generated during welding or thermal cutting processes. The temperature rise changes the microstructures of the HAZ but is insufficient to melt the parent metal. HACC does not necessarily occur immediately after welding, but often develop while in storage or in service. HACC will occur only if the necessary conditions are met. 2.1.1 Factors Influencing HACC The susceptibility of a weld to HACC is dependent on the amount of hydrogen within the weld. In general, the risk of cracking is greater when the amount of hydrogen present in the weld increases. When assessing the possibility of HACC to occur in a steel weldment, three important factors are considered: 1. Presence hydrogen in the weld 2. Sufficient stress (applied or residual) acting on the weld 3. A susceptible microstructure is present. 2.1.1.1 Sources Of Hydrogen It is necessary to assume that presence of hydrogen in weld is inevitable and hydrogen is always present during welding. Atomic hydrogen is absorbed into the weld pool during welding as molten steel has a high solubility for hydrogen. As the weld cools down, some hydrogen escapes from weld pool as the solubility of weld decreases. The escaping hydrogen is called as diffusible hydrogen while the hydrogen remaining in weld is called 3 as residual hydrogen (Smialowski, 1962). The amount of diffusible hydrogen is dependent on the original amount of absorbed hydrogen, the size of the weld, the level of solubility and the time-temperature conditions of cooling (Bailey, 1974). The solubility of hydrogen in weld as a function of temperature can be seen from fig 2. Fig 2. Solubility Of Hydrogen With Respect To Temperature Variation For A Typical Weld Metal The atomic hydrogen will diffuse into areas where vacancies are present and will combine with other atomic hydrogen in order to lower its Gibb's Free Energy. The molecular hydrogen tends to migrate towards voids in the material where they form small pockets of gas, which will lead to confined areas of high pressure. As the amount of hydrogen present increases in these areas, pressure also increases. The amount of hydrogen involved in a welding process can be controlled by various means. Implementation of control procedures by minimizing hydrogen absorption and ensuring sufficient diffusion before the weld cools can reduce the amount of hydrogen in a weld. Fig. 3 shows the diffusion rate of hydrogen through ferritic steel. 4 Fig 3. Diffusion rate of hydrogen through ferritic steel decreases as temperature falls. Identifying and controlling the sources of hydrogen can also achieve reduction in hydrogen absorption. Three sources of hydrogen that must be taken into consideration are: 1. The welding consumable 2. The atmosphere 3. The parent steel For most instances, the most important source of hydrogen is the welding consumable’s coating (Smialowski, 1962); however sometimes large amounts of hydrogen originated from one of the other sources could have a more substantial effect. 2.1.1.1.1 Hydrogen From The Welding Consumable A major proportion of hydrogen in the weld metal is derived from moisture, combined water and other hydrogenous compound in the consumable’s coating. Dissociation of these elements by the welding arc produces large quantities of atomic hydrogen that can dissolve into the weld pool. Proper storage of consumables helps to reduce moisture absorption. When required, the consumable needs to be dried of baked to lower the moisture content for producing welds with minimal amount of hydrogen. The content of 5 hydrogen in flux-cored consumables may range from very low to medium and some may have properties such a resistance to moisture absorption. Hydrated oxide, e.g. rust, on the surface of solid wire could contribute to high level of hydrogen in welds even though low hydrogen levels are normally achievable from solid wires. 2.1.1.1.2 Hydrogen From The Atmosphere The welding arc can break down moisture from the ambient atmosphere into hydrogen that can be readily absorbed into the weld pool. This source of hydrogen should not be overlooked especially when welding is done in a humid environment (Bailey, 1974). 2.1.1.1.3 Hydrogen From The Parent Steel The presence of paint, oil, grease, rust and other contaminants on the surface of steel can be broken down by the welding are to produce hydrogen that could be absorbed into weld pool. Steels exposed to an environment of high temperature and high concentration of hydrogen for a considerable length of time is likely to have high hydrogen content as a result of absorption. Hot working or heat treatments is able to diffuse out hydrogen and lowers the content of hydrogen, making the steel safe to be welded without HACC. 2.1.1.2 Stress (Applied or Residual) Welding can cause distortion and create stresses in the weld. The intense heat of the welding arc can cause localised and non-uniform heating of the parent plate. Hence when welding is in progress, the area of the parent plate next to the arc will experience rapid heating and then cools down as the welding torch moves away. Stresses are induced by thermal expansion and contraction of the weld metal and HAZ. The unheated area of parent plate imposes a restraint on the above, and as contraction predominates, the weld metal cannot contract freely; therefore stress is built up in the joint. This stress is known as residual stress. Residual stress up to the yield strength of the weld metal is unavoidable in all welded structures. The effect of residual stress can be intensified by stress concentrations and these are areas where cracking would first appear. Distortion of the 6 weldment would only happen if proper control procedures were implemented. Control such as welding technique, jigs and fixtures, fabrication procedures, and final heat treatment, vibratory stress relief or peening are able to reduce or remove residual stress. Fig 4 and 5 shows residual stress distributions. Fig. 4 Residual stress distributions: (a) longitudinal stress in single-pass weld: (b) transverse stress in single-pass weld 7 Fig 5 distribution of residual stress along a weld corresponding to the temperature change External loads on the welded structure create different forms of stress on the welded joint. When the load is sufficiently high, the applied stress would allow HACC to occur. 2.1.1.3 Susceptibility Of Microstructure The susceptibility of steel to HACC is generally dependent on the inherent toughness of the steel. The possibility for HACC to occur increases as the hardness of steel increases or as toughness decreases. As expected, for tougher steel, more hydrogen would necessary to be present in order for cracking to occur. 2.1.1.3.1 Carbon Equivalent When cracking occurs in steel, it often suggests that the steel is susceptible. The term susceptibility is often related to brittleness or hardness. As the hardness of steel increases, it is generally accompanied by a reduction in its toughness. Usually, the crack will propagate along a path where the steel is most brittle or less tough. The Carbon 8 Equivalent, CEIIW formula is frequently used to correlate the hardenability or susceptibility of steels with a carbon content in excess of 0.18% where CE IIW C Mn Cr Mo V Ni Cu 6 5 15 The alloying elements taken into consideration in this formula include carbon (C), manganese (Mn), chromium (Cr), molybdenum (Mo), vanadium (V), nickel (Ni) and copper (Cu) (Lancaster, 1980). This formula employs the principle of comparing the contribution of each of the alloying elements in steel to the hardening effect to the amount of carbon alone that would invoke the same effect. The carbon equivalent of an alloying element is obtained by dividing the amount of the alloy present in steel by a factor. Therefore, it can be deduced that higher value of carbon equivalent corresponds to the greater degree of hardness of the steel. Fig. 6 Comparison Of Tensile Strength Against Carbon Equivalent Figure 6 shows the comparison between carbon equivalent and ultimate tensile strength for normalised and tempered (N and T) and quenched tempered steel (Q and T). The 9 comparison as depicted in figure 6 is more appropriate for steels with low alloying composition. Steel with carbon equivalent values above 0.45 is more susceptible to hydrogen cracking while having values below 0.42 imply that steel has good weldability, without being subjected to the risk of hydrogen cracking (Bailey, 1994). Carbon equivalent is a general method used to determine if steel is susceptible to HACC. However the conventional way of calculating the carbon equivalent based on the nominal composition of the weld metal may be inappropriate. This can be illustrated in cases where segregation occurs in the weld metal. Even though the calculated carbon equivalent based on the nominal composition of the weld metal is below 0.42, high concentration of alloying elements trapped in the grain boundaries may significantly increase the carbon equivalent value to that higher than 0.45. As such, in regions where segregation occurs, the weld is more brittle and prone to hydrogen cracking. This implies that segregation may be a factor that influences the susceptibility of welded steel to hydrogen cracking. Toughness of steel can also be related to the type microstructure which the steel is made up of. The crystals in steel are known to undergo transformation when they are subjected to high temperatures, beyond the crystallisation temperature. This happens especially during welding when the arc temperature could reach 5,ooo to 30,000 degrees Celsius. Coarse crystals are developed in the HAZ near to the fusion boundary that is exposed to temperatures around 1,500 degrees Celsius. Fast cooling rate from the maximum temperature will produce martensitic microstructures. This microstructure is very hard and has poor toughness. Slow cooling rate however will produce coarse microstructures largely of bainite, which although softer than martensite, is often less tough. In general, when the rate of cooling is sufficiently fast, fine crystals will be formed while slow cooling rate will produce coarse crystals. Fine crystals exhibits better mechanical properties such as strength and toughness, than coarse crystals which are often more brittle. 10 2.1.1.3.2 Susceptibility Of Different Steel Types Generally, the composition of alloying elements is one factor that affects the mechanical properties of steels. Steels with high alloying content have high strength and hardness. However, toughness of steel is inversely proportional to its strength and hardness. This is the reason steels with high alloying content such as cast iron are often brittle. Below is a list of different steel types and their levels of risk to HACC respectively. Mild Steel: The possibility of hydrogen cracking is low with the material thickness below 50mm, unless the material is subjected to very high restraint Carbon, manganese steels: Likely to suffer from hydrogen cracking, but are otherwise similar to mild steel Microalloyed weldable steels: Resistance to hydrogen cracking is influenced by carbon content Quenched and tempered weldable steels: Hydrogen cracking may occur for weld metal with low carbon content Low alloy weldable (pressure vessel) steels: Preheating is required to prevent hydrogen cracking Engineering steels (medium to high carbon, with or without alloying elements): High risk of hydrogen cracking Tool steels: Extremely high risk of hydrogen cracking (Bailey, 1994) 2.1.2 Important/characteristic Of HACC Major problem in industry where substantial costs are incurred for the repair of welds resulting from HIC Cracking is difficult to detect because it does not occur directly after the welding operation, rather it occurs hours or days afterwards Suddenly fail without warning –cracks may be undetectable via visual inspection if cracking occurs in the bulk of the material which due not propagate towards the surface. 11 According to Steinberger and Stoop (1952), a study on the crack sensitivity of welded metal aircraft steels led to the following conclusions: Stress alone did not cause cold cracking. Hydrogen free deposits did not crack even under high stress. Weld deposits become increasing sensitive to stress as the amount of hydrogen is increased. Weld deposits essentially free of hydrogen did not crack even at high cooling rate resulting from welding while quenching with water. When a small amount of hydrogen entered the weld zone, high cooling rates cause cracking. From Steinberger and Stoop (1952) findings, it can be concluded that the HACC will only happen if the hydrogen and susceptible microstructure is present and sufficient stress is applied to weld. The HACC will not occur if one of the above criteria is not present, however it practical, those three criteria always present simultaneously. This means that HACC is unavoidable under normal welding practice. If the hydrogen level in weld metal is sufficient, stress exist in the weld, residual or applied or both, and the susceptible of weld microstructure is present, then the development of cold crack or ‘fisheye’ will occur. The term fisheye was introduced to designate bright spot observe in fractures surfaces of weld deposit. A fisheye usually surround some kind of discontinuity in metal such s a non-metallic inclusion, or gas pocket, which probably acted as a nucleus for the formation of actual “eye”, refer to Fig .7. From the previous studies showed that these defects are due to hydrogen. 12 Fig. 7 Fisheye On The Fracture Surface 13 2.2 Solidification Of Fusion Weld 2.2.1 Microstructure Of Metals Solid metals are crystalline. When molten weld metal cools down to a specific temperature, the solidification process will occur. During solidification or crystallisation, the free moving atoms of the molten metal begin to slow down and arrange themselves according to some regular pattern or ‘lattice structure’ (Higgins, 1987). The regular pattern or ‘lattice structure’ is actually the geometrical feature possessed by the crystal. Throughout the entire process of solidification, energy will be liberated continuously as latent heat. (Henry & Claussen, 1940) Detailed study of the solidification process under microscopic observation revealed that upon reaching the freezing temperature, some group of atoms from the molten metal will position themselves to form a minute crystal called the nucleus. Each nucleus manifests itself as an individual crystal lattice unit. The crystal grain ‘grows’ as more atoms attach themselves to the nucleus and the rate of growth is influenced proportionally by the directions where heat is drawn from the melt most rapidly. The crystal nucleus has the appearance of a dendrite or having resemblance to a pine tree. The arms of the dendrite or the branches of the pine tree grow continuously until they touch the outer arms of other similar dendrites growing within neighbouring surroundings, thus defining a boundary between the dendrites (Higgins, 1987). When the outward growth of the dendrite is being arrested, the existing arms would then get thicker. In this manner, the cavities in between these arms will be filled up. The growth of these dendrites is allowed with the availability of space and also continuous supply of molten metal in the surrounding. When the molten metal is absent, the growth of dendrites will cease and creating possibility for formation of cavities between the dendrite arms. Fig. 8 shows the way a crystal grows with dendrite extending in the direction of heat dissipation. 14 Fig. 8 Growth of a Crystal All crystal grains grow independently in random order without any orientation. Coupled with the event where the arms of a dendrite grow at different rates and the outer arms of neighbouring dendrites making contact at random angles, irregular shaped grains with curved boundaries are the result. Under high magnification, it can be observed that the surface of a solidified metal has crystal grains bounded by irregular boundaries connected to each other (Higgins, 1987). Fig. 9 shows the various stages of solidification of a metal. 15 Fig.9 The Dendritic Solidification Of A Metal The rate of cooling of the molten metal is one factor, which controls the size of the crystal rains. Rapid cooling is usually accompanied by the formation of more nuclei and hence produces fine sized crystal grains. Smaller crystal grains means more grains presence in the solid. In contrast, a slower cooling rate will give rise to larger and fewer grains presence in the solid (Henry & Claussen, 1940). Evidently, if cooling occurs from a point much higher that the freezing point, a longer time would be needed for the temperature to drop to a point for solidification to begin and thus, larger crystal grains would eventually be formed. It is undesirable for coarse grains to be present in a solid since it has poor mechanical properties such as brittleness. On the other hand, a solid with fine grains tends to display greater strength and toughness than the one with coarse grains. 16 Iron has polymorphic properties; this means that iron exhibits different crystal structures at different temperatures. It exists as body-centred cubic form (alpha iron) below 910 degrees Celsius and face-centred cubic form at elevated temperatures beyond 910 degrees Celsius (Higgins, 1987). Fig. 10 shows a body-centred cubic structure. Fig. 10 A Body-Centred Cubic Structure 2.2.2 Formation Of Solid Solution In Weld Very often in metal works, alloying is the preferred way for enhancing the properties of a metal. The usual practice of alloying is to add other elements to the base metal. For example, minute amount of alloying elements such as Chromium (Cr), Nickel (Ni), Molybdenum (Mo), Vanadium (V) and Manganese (Mn) are added to iron to produce low alloy steels, which is tougher than pure iron. When welding is performed, the electrode is melted and mixes with the steel from the parent plate to form the weld metal. The introduction of the molten electrode to the weld pool increases the content of alloying elements and changes the chemical composition of the weld metal, making it different to the composition of the parent plate. A solid solution is obtained when two or more elements remain dissolved in each other after solidification. However, as in this and most cases, due to differences in compositions and melting points, one element may solidify more rapidly than the other and a phenomenon known as coring may occur in 17 this instance. As a result, crystals are formed with cores having high content of the elements with higher melting point, whereas the outer fringes are richer in the element with lower melting point. Subsequently, additional heat treatment processes such as annealing can be performed in this case to enable diffusion and to create a uniformly distributed crystal structure. 2.2.3 Microsegregation In Weld In a steel welding process, the electrode is melted under the high temperature of the arc and is deposited in the weld pool. Subsequently, dilution occurs when a proportion of the parent metal melts and is incorporated into the weld pool, which later solidify to form the weld metal. Hence, the elements that constitute the electrode are introduced and mix with the parent metal. In instances where these elements are unable to form a solid solution with the parent metal, they may exist as individually independent crystals upon solidification. Rapid rate of solidification coupled with the differences in melting point and composition of alloying elements may cause them to remain in solution until solidification was almost complete. They would therefore remain concentrated in that metal which solidified last; that is, between the dendrite arms and is termed as microsegregation in the microstructure. In this manner, microsegregation may occur parallel to the direction of solidification. The existence of microsegregation reveals a dendritic pattern when a suitably prepared specimen is viewed under the microscope. Microsegregation at crystal boundaries due to accumulation of alloying elements weakens the solid and have devastating effect on mechanical properties. This offers an explanation why the solid is likely to fail along the crystal boundaries where microsegregation occurs (Higgins, 1987). Refer to Fig .11 Microsegregation occurring at crystal boundaries. 18 Fig .11 Microsegregation Occurring At Crystal Boundaries 2.2.4 Macrosegregation In Weld In addition to microsegregation at all crystal boundaries, macrosegregation occurs at a larger scale. As relatively steel crystallises during the early stages of solidification, the ‘impurities’ are left undisturbed and there is a general accumulation of alloying elements in the centre region of the weld pool. This is where metal solidifies last of all, and has become most charged with those elements. In this manner, macrosegregation occurs perpendicular to the direction of growth of crystals. 19 3. Part 1 – Welding In this chapter, issues related to welding that were considered in this project will be discussed in detail, covering various aspects ranging from the selection of appropriate welding process, to problems that were encountered during welding. The welding technique employed throughout the entire project is based on information available from welding handbooks, journals, manuals, work standards and also literatures. However, in order to achieve good and consistent results, the technique was improvised regularly for optimisation. This was made possible with gradual acquisition of experience and advice from personnel who have profound knowledge in welding. 3.1 Welding Process 3.1.1 Process Selection Steels are usually welded using arc welding. A few examples of arc welding process are shielded metal arc welding (SMAW), submerged arc welding (SAW), gas metal arc welding (GMAW), gas tungsten arc welding (GTAW) and flux cored arc welding (FCAW). In the early stages of the project, discussions were carried out to evaluate all options prior to the selection a suitable welding process. SAW is eliminated from the selection process, as it is unavailable. SMAW and GTAW are not favourable because these processes require a high level of workmanship and experience. GMAW and FCAW are semi-automatic welding processes that offer high metal deposition rate and efficiency. High level of metal deposition enables rapid welding to be carried out and will result in minimum distortion and a narrow heat-affected zone. In the end of the selection process, FCAW is chosen because it was found from the previous research, HACC would likely occur at weld produced using the FCAW process instead of GMAW process. Welding will be conducted in the Mechanical Engineering workshop with the aid of an automated welding machine. 20 Initially, a series of trial welding was conducted on AS 1018 steel plates for familiarisation with the welding process and derivation of the optimum welding conditions. When these have been achieved, actual welding would then be performed on X80 steel under the optimum welding conditions that have been identified previously. This will minimise unnecessary wastage of X80 steel that is more expensive than the carbon steel plates. 3.1.2 Fundamentals Of Process The consumable electrode used in a FCAW process is in the form of wire. This consumable is fed from a spool through the conduit (cable) then through the welding torch to the arc point. As the wire passes through the contact tube in the welding torch, it picks up the welding current. Coalescence of metal is produced by heating the wire with an arc between wire and base metal, refer to figure 12. Gas-shielded type of FCAW is similar to GMAW. The main different between these two processes is the consumable used; FCAW uses flux-cored wire consumable while GMAW uses solid wire consumable. 21 Fig. 12 Gas-Shielded FCAW 3.2 Material 3.2.1 Grade AS 1018 Carbon Steel Grade AS 1018 carbon steel plates were used as the base metal in trial welding. The plate’s dimension is 2506510 mm. The chemical composition and mechanical properties are shown in table 1 and table 2 Material AS 1018 C Si Mn 0.18 0.25 1.0 P S 0.006 0.023 Cu Ni 0.23 0.08 Cr Mo V 0.05 0.017 0.007 Table 1 - Typical Chemical Composition (%Wt) Of Grade AS 1018 Steel Material AS 1018 Minimum yield Minimum tensile Minimum strength (MPa) strength (Mpa) elongation (%) 320 440 22.0 Table 2 - Mechanical Properties Of Grade AS 1018 Carbon Steel 22 3.2.2 API 5L X80 Steel X80 is High Strength Low Alloy (HSLA) steel, which commonly used as line pipe steel. The high yield strength of X80 means thinner steel can be used to meet the stress requirements. Thinner steel, in addition to requiring less steel, demands less welding time, thus inducing cost saving. Thermo-Mechanical Controlled Processing (TMCP) is applied to incorporate better mechanical properties to the steel through refinement of the steel microstructures. The chemical composition and mechanical properties are shown in table 3 and table 4. The most problematic aspect was that of achieving the same yield strength in the weld as in the plate. An elaborate heat treatment cannot be performed on a weld metal as it can with plate, so to match the plate strength the weld metal has to rely on alloying additions. This, however, increases hardenability with the associated increase in risk of weld metal hydrogen cracking. Material C API 5L Si Mn P S Cu 0.11 0.16 0.74 0.013 0.002 X80 Ni 0.14 0.75 Cr Mo V 0.50 0.43 0.05 N 0.004 Table 3 - Typical Chemical Composition (%Wt) Of Grade X80 Steel Material API 5L X80 Minimum yield Minimum tensile Minimum strength (MPa) strength (MPa) elongation (%) 584 679 46.7 Table 4 - Mechanical Properties Of Grade X80 Steel 23 3.2.3 Flux Cored Wire It is very important to select a consumable that is compatible with the base metal and welding process. For gas-shielded FCAW process with steel as the base metal, gasshielded steel flux cored wire should be used. If the wire is incompatible with the base metal, the quality of welded produced will low as can be observed when an aluminium typed wire is used to weld steel base metal. In addition, the chemical composition of the selected wire should (near) be the same as the chemical composition of the base metal. The consumable diameter is dependent on the thickness of the base metal. In this project, a 1.2 mm Fluxofil 20H5 is used. This consumable is a tubular flux cored wire that is rutile based and meets the Australian standard AS 2203.1 classification Etp_Gmp-W559A.Ni1 H5. Nickel contained in the electrode is deposited into weld pool and thus incorporating high strength with excellent low temperature impact properties into the weld metal. Selection of the Fluxofil 20H5 consumable is primarily due to compatibility between the X80 steel and the consumable, which is necessary in order to produce a weld with properties similar to the base metal. This wire is suitable for all position fillet and buttwelding application to produce smooth arcing and good weld bead profiles. It is used with an argon based shielding gas with 15 to 25 % carbon dioxide. Flux cored wire produces slag that is fast freezing, can be easily controlled and has low spatter levels. The composition and mechanical properties of the wire are show in table 5 and table 6. The data sheet for Fluxofil 20H5 is included in the appendix. Compound C Mn Si Ni B Fe Fluxofil 20H5 0.05 1.1 0.55 0.9 0.004 Bal Table 5 – Composition Of The Flux Core Wire YIELD STRESS TENSILE STRENTH ELONGATION CVN IMPACT VALUES 590 MPa 640 MPa 24% 55J @ - 30 C Table 6 – Typical mechanical properties of weld metal 24 25 3.3 Equipment The figure 13 below shows the equipment used in the welding process. Speed Controller Carriage Shielding Gas Welding Torch Base Supporter Welding Machine Fig. 13 Welding Equipments Switch 3.3.1 Welding Machine The welding machine used in this project is PROMIG 500 welding machine made from Kemppi OY Company. It consists of wire feeder, main power source and main control panel, refer to figure 14. 26 Main Control Wire Panel Feeder Power Supply Fig. 14 – PROMIG 500 The wire feeder system continuously feeds the consumable wire from spool to the welding arc through welding torch at a constant speed, refer to figure 15. The wire-feed speed can be adjusted from the main control panel. When the wire-feed speed is set at a specific rate, a proportionate amount of current is automatically drawn. Which mean the current of welding process is based on the wire-feed speed. When the wire-feed speed is faster, the current increase, likewise the current decrease if the wire-feed speed is slower. 27 Fig. 15 Wire-Feeder Machine Main power supply provides DC constant voltage (CV) power sources to the welding process. The CV type power source has a nearly flat volt-ampere characteristic, which mean the voltage is stay constant regardless of the amount of current used. The voltage depending on the arc length, whenever the nozzle-to-work distance changes, the arc length and the voltage changes. This type of power source is important to produce uniform welds by maintain the arc length and voltage at a constant value. Also with this type of power source, the wire-feed speed can change over a considerable range without causing the wire to burn back to the nozzle. The welding machine main control panel, refer to figure 16, provide user to control over the welding process. For example Controls and displays of main welding parameters (wire feed speed or current, voltage, welding dynamics, plate thickness) Selection for controls: local controls, gun remote control unit, and remote control unit 28 Cable and gun lengths of welding circuit can be taken into consideration by the calibration function Fig. 16 Main Function Panel For detail information of PROMIG 500 machine, please refer to appendix y. 3.3.2 Carriage The welding process of this project will be assisted by mechanism device to become a fully automated welding process. The mechanism devices is named as carriage, which are able carry a welding torch and move in linear path at constant speed, refer to figure x. The carriage is able to move horizontally in both forward and reverse directions with different speed settings. The speed can be adjusted by turning the knob on the carriage speed controller. A torch holder was designed and fabricated to hold the welding torch. The torch holder is designed to allow for adjustment of the distance and angle of the torch relative to the work piece. The fabrication drawings of the torch are available in appendix B. 29 Fig. 17 Carriage Fig. 18 Carriage Speed Controller The average time taken by the carriage to move a distance of 150mm was measured. This enables the speed to be determined with respect to the different settings of the controller from ‘1’ to ‘9’. Forward direction [s] Knob 1st try 2nd try Ave. 1 61.21 60.06 60.64 2 42.09 42.41 4 35.62 5 Reverse direction [s] Speed Speed 1st try 2nd try Ave. 2.47 74.96 76.21 75.59 1.98 42.25 3.55 49.90 48.49 49.20 3.05 35.91 35.77 4.19 38.03 38.04 38.04 3.94 33.30 33.15 33.23 4.51 34.54 34.33 34.44 4.36 6 33.16 33.10 33.13 4.53 34.30 34.35 34.33 4.37 8 37.80 37.80 37.80 3.97 41.33 41.05 41.19 3.64 [mm/s] [mm/s] 30 9 33.00 33.20 33.10 4.53 34.73 34.20 34.47 4.35 Table 7 - Speed Of The Carriage For Different Speed Knob Number From the table 1, it can be observed that the speed of the carriage does not change proportionally with respect to the settings. The table also shows that the speed of the carriage for the same speed setting differs when it is moving in different directions. The maximum speed attained by the carriage in the forward direction is 4.5mm/s and 4.3mm/s in the reverse direction, when the speed setting is ‘5’. There is no significant speed increment for any settings above ‘5’. This indirectly implies that the device is not well designed. Calibration of the device was performed regularly to ensure that there is no deviation so that the results would be consistent. 3.3.3 Shielding Gas The shielding gas plays an extremely important role in this welding process. The purpose of the shielding gas is to prevent contamination of the electrode and weld pool by air (nitrogen and oxygen). It also provides a local atmosphere to maintain a stable arc. Inadequate shielding will result in porous and brittle welds. It is important to select the correct shielding gas. The shielding gas is selected according to the type of metal being welded. Selection of the wrong gas for the metal to be welded can result in porosity, brittleness, and/or undesirable penetration of the weld. For steel, a gas mixture of 75% argon and 16% - 25% carbon dioxide is used as shielding gas. This gas mixture helps to prevent burn-through and distortion on very thin steel, yet provides good penetration on thicker steel. Its ability to minimize spatter results in clean, smooth weld appearances. The amount of shielding gas (flow rate) is also important, the proper amount of gas shielding results in a rapidly crackling or sizzling arc sound. Inadequate gas shielding produces a popping arc sound and results in weld discoloration, porosity, and spatter. In this process, shielding gas is supplied by the BOC Gas Company. The shielding gas is 31 Argoshield Universal (Industrial Grade Gas Code No. 065) and has the following composition: Gas Purity Oxygen 2.75% Carbon Dioxide 16% Argon Balance Table 8 – Composition Of Shielding Gas Used The flow rate of the shielding gas was set to 20 litre/min. This is the recommended flow rate of shielding gas for the selected welding process, as mentioned in the data sheet of the consumable. The data sheet is available in appendix a. 3.3.4 Welding Torch The function of the welding torch is to deliver the electrode wire, the welding current, and the shielding gas to the arc area. The wire is fed to the torch by an automatic wirefeeder machine, which pushes the wire through a flexible tube inside the welding cable to the arc point. The gun has a trigger switch that controls the wire feed. There are two modes of trigger, which can be selected from the main control panel. The first mode is 2T whereby the wire feeder is activated once the trigger switch is pressed. The feeder is turned off when the trigger switch is pressed for the second time. The second mode is 4T, which requires welder to press and hold the trigger switch to activate the wire feeder. If the trigger switch is released, the wire feeder is deactivated. In this project, 4T mode is selected to enable the operation of the remote trigger switch. Instead of the torch trigger switch, the remote switch is used for initiating and terminating the welding process. This is because of the difficulty and danger that arises in the attempt to reach for the trigger switch of the torch while the torch is constantly in motion when welding is in progress. 32 Fig. 19 Switch The welding gun used in this project is BEQA310A-E Bernard 300 Amp Gun cable, refer to figure x: The specification of the torch is listed below: Light weight for light to medium fabrication applications. Duty cycle: 200 Amp @ 100% with mixed gas. Euro Fitting Meter long cable Straight Body tube - 125mm BEQT5-180 3.3.5 Base Support Two plates are placed flatly on to a base support, with a gap of 1.5mm along the length, as required for butt weld. The flat and level surface of the support is crucial for maintaining the alignment of the plates. Misalignment of the plates has a detrimental effect on the quality of the weld, as well as inducing stress along the weld. This is undesirable, as it affects the microstructure of the plates. A clamping jig that consists of two solid square bars is used to restrain both plates in position during welding and cooling cycles. Tightening of the cap screws located at each end of the bars will produce 33 the clamping force on the plates. The clamping jig is designed to be heavy so that the jig will remain stationary and not be affected any vibration that might be resulted from moving parts when welding is in progress. This helps to create a stable welding process. 3.3.6 Personal Protective Equipment Most welding processes are associated with hazards. These hazards include electric shock, burns, fire and explosion, radiation, heat, noise, fumes and gases. While welding, a welder is always exposed to health risks and therefore safety is extremely important. In this project, strict safety procedures are practiced and no compromise is accepted. Safety checks are performed routinely to make sure that equipment is in proper working order. Welding is always carried out with care and it is compulsory that protective clothing, such as long sleeved shirt, long pants and gloves, is worn. Proper eye protection is used to shield off glare and radiation. A ventilation hood keeps the welding area well ventilated as well as keeping fume in low concentration. All flammable materials are removed from the welding area to reduce fire risks. Curtains are used to ensure that other people in the vicinity are not offended or irritated by flashes of the arc. The floor is kept dry at all times in order to minimize the possibility of electric shock. 3.4 Welding Conditions 3.4.1 Joint Geometry Flat position open square butt joint geometry is chosen for all welds produced. A gap of 1.5 mm between the 2 abutting edges of the plates is maintained for all welds Single pass welding is performed on both sides of the plates along the edges parallel to the rolling direction of the plate. Half joint penetration is achievable using this configuration. 34 3.4.4 Electrode Polarity There are two types of electrode polarity for the DC source, namely direct-current reverse polarity (DCRP) and direct-current positive polarity (DCSP). Under DCRP mode, the consumable is connected to the positive terminal while the clamping jack is negative. The configuration of both settings is shown in figure 20., DCRP is more desirable in FCAW processes as deeper penetration and excellent cleaning action can be achieved. Fig. 20 Electrode Polarity 3.5 Welding Parameters 3.5.1 Welding Current As mentioned previously, the welding current changes with the variation of the wire-feed rate. This adjustment is limited to a definite range, depending on the welding current used. The welding current changes proportionally to the electrode feed rate. It is also dependent on the type of electrode, its diameter and the length extended from the tip of the torch. Variation of the welding current will produce the following effects: Increase in electrode deposition rate as welding current increases Increase in penetration as welding current increases. Excessive current produces convex weld beads with poor appearances. Insufficient current produces large droplet transfer and excessive spatter. As welding current is increased or decrease by changing electrode feed rate, power supply output voltage should change to maintain the optimum relationship of arc voltage to current 35 3.5.2 Arc Voltage Arc voltage and arc length are closely related. Arc voltage can affect the appearance, soundness and properties of welds produced with flux-cored electrodes. Either too high an arc voltage or too long an arc will result in excessive spatter and wide, irregular shaped weld beads. However, too low an arc voltage or too short an arc will result in narrow convex beads with excessive spatter and reduced penetration. 3.5.3 Electrode Extension In FCAW process, wire stick-out or electrode extension refers to the distance the wire extending from the tip of the torch, refer to figure 21. A correct electrode extension is necessary because it influences the welding current. The current output is reduces automatically when the electrode extension is increased, and vice versa. Too much extension produces an unstable arc with excessive spatter, while insufficient extension causes excessive arc length at a particular voltage setting and fusion of the wire to the nozzle tip, which decrease the tip life. 36 Fig. 21 Electrode Extension 3.5.4 Travel Speed Travel speed of the welding torch can influence weld bead penetration and contour. While holding other factors constant, better penetration is achievable at low travel speed as compared to high travel speed. Over-heating of weld metal occurs when the travel speed is too low. As a result, the weld will have a rough appearance and yields possibility if mechanically trapping slag. A torch moving too fast will result in irregular, ropy appearing beads. The width of HAZ can be controlled by the travel speed. By increasing the travel speed, the HAZ width can be reduced. In addition, travel speed can also affect the weld bead profile. Fig 22. shows weld bead profiles obtained from different travel speed. 37 Fig. 22. Formation of weld bead profiles for (a) slow travel speed and (b) fast travel speed. 3.5.6 Electrode Angle Proper positioning the welding torch and weldment are important. The orientation of the torch in relation to the plate is called the work and travel angle, refer to figure 23. For this project, which the base metals are equal in thickness, the work angle should normally be on the centerline of the joint. The travel angle refers to the angle in which welding takes place. For gas shielded FCAW processes, this angle should be between 5 to 15 degrees. If the angle is too large, the effectiveness of the shielding gas will be lost. 38 Fig. 23 Travel And Work Angle The travel angle may be either a push angle or a drag angle, depending on the position of the torch. Pulling, dragging or backhand technique is a technique when the torch is ahead of the weld. When the torch is behind the weld, it is referred to as pushing or forehand technique. The drag technique produces greater penetration than the pushing technique. Fig. 24 Welding Techniques 39 3.5.7 Heat Input The heating produced by welding is normally described in terms of the heat input per unit length of weld run, usually in units of kJ/mm if the welding speed is measured in mm/min. Heat input, E indicates the amount of heat supplied to the parent metal by the welding process. It is derived from the arc energy which is calculated from the welding current, arc voltage and welding (travel) speed as follows: E V A 60 KJ / mm 1000 S where V = arc voltage A = Welding current S = Welding speed or travel speed [mm/min] In both fusion and non-fusion welds, the need to provide adequate heat for fusion must be balanced against the detrimental effects of heating. To reduce the hardness level of the steel below a critical value in order to avoid cracking, it is necessary to control cooling by adjusting the temperature of the steel being welded, i.e. preheat and also the amount it is heated during welding by adjusting the heat input of the welding process. Heat input can also impose an influence over the HAZ width. A lower heat input will produce a narrower HAZ. 3.6 Welding Procedures The equipment is set up according to discussion on previous sub-chapters. Refer to figure 13 – overview of welding equipment 1. The surfaces of the base metal are cleaned from contaminants such as grease oil, dirt and etc. 40 2. Run on and run off tab is welded to edge of the base metals to restrain the plates for attaining a consistent joint gap during welding. Tabs on both sides of the plates. 3. The plates are clamped on to the base support and trial run is done to get good alignment between gap of base metal and welding torch. 4. Welding is initiated at the run on tab and ends at the run off tab. 5. The slag is removed from the weld surface before another welding is continued on the other side. 6. The welded plates are left to cool at room temperature. 3.7 Discussions In this section, results obtain from the welding process, together with problems and difficulties encountered along the way will be discussed. 3.7.1 Test/Trial Run The following table 9 shows a summary of the conditions used in the welding process. In addition, table y shows the welding parameters used and the associated values heat input. Process Gas-shielded FCAW Joint design Double side, single pass, flat square butt joint with 1.5 mm gap Shielding gas Argoshield Universal Base Metal X80 and Black Flat Bar Electrode Polarity Reverse polarity Electrode angle Drag angle – 5-10o Electrode Extension 19 mm 41 Welding current 180 –250 A Arc voltage 26-29 V Travel speed 150-250 mm/min Table 9 – Summary Of Welding Conditions Arc Voltage Welding [V] [A] [MM/min] [KJ] FCAW-Run1 28 200 150 2.2 FCAW-Run2 28 250 300 1.4 Descriptions Current Travel Speed Heat Input Table 10 – Values Of Heat Input Corresponding To Welding Parameters 3.7.2 Lack Of Penetration This type of imperfection is commonly found in consumable electrode processes such as FCAW, where the weld metal is 'automatically' deposited as the arc consumes the consumable. The welder has limited control of weld pool penetration independent of depositing weld metal. Several known situations that could lead to this type of imperfection and its associate corrective actions are as follow: Situations Wrong welding parameters Misplaced weld Joint gap closing when welding Actions Trial and error using optimum value refer to handbook Few carriage run along the joint gap to get a good alignment Adequate tacking is use 42 3.7.3 Surface Porosity Figure 25 below shows one of the defects encountered during the welding process. This defect is identified as the surface porosity. Surface porosity is resulted from atmospheric contamination. A clogged nozzle, shielding gas set too low or too high, or welding in a windy area can cause surface porosity to occur. After analysing the possible causes to this problem, it was found that the nozzle nose was clogged. Accumulation of spatter particles at the nozzle nose restricts the flow of shielding gas, hence reducing the efficiency in the delivery of the gas to the weld poll. As a consequence of the blockage, the molten weld metal is contaminated by atmosphere due to insufficient shielding. Corrective action was taken by removing the spatter particles from the nozzle and problems related to surface porosity have been successfully eliminated. Figure 25 –Surface Porosity 3.7.4 Reduction Of Joint Gap It was mentioned previously that the purpose of tacking is to maintain the joint gap. However after the first pass (one pass on each side is required), the gap on the other side is reduced significantly due to shrinkage of the weld metal. The initial joint gap of 1.5mm has reduced to less than 1mm. This undesirable as a narrow gap inhibits penetration of a weld. Distortion is identified as the cause to this problem. Thermal stresses induced by expansion and contraction of the weld pool into base metal cause volume change. The main type of distortion of causing the reduction of joint is transverse shrinkage. The amount or/and quality of tacking is insufficient to restrain the shrinkage of the base metal. The figure 25 below show the example of the insufficient of tacking, the tacking is not 43 ‘strong’ enough to resist the transverse shrinkage force thus break. A corrective action of instead of single tacking, a continuous tacking is used. By using new method of tacking, the amount of gap reduction is reduced. It is impossible to maintain the gap at 1.5mm, thus the pre-weld joint gap is increase few mm to counter the amount of gap loss by shrinkage problem. 3.7.5 Weldability Weldability is defined as the ability to produce a weldment on a metal with properties that could fulfil the requirement of an application and yet the weldment should not have any discontinuities, such as cracks or other detrimental defects. Other definitions of weldability are the ability to avoid the formation of martensite or possessing a value of carbon equivalent lower than a certain value. The content of carbon in the composition of weldable steels is generally low that is less than about 0.2% and this amount reduces as the content of other alloying elements increases. The calculated value of carbon equivalent for API 5l grade X80 steel based on its composition is 0.38 which is lower than 0.42 and this literally imply that this steel can be welded without hydrogen cracking. As dilution is expected to occur with the introduction of the parent metal into the weld pool, thus the value of carbon of carbon equivalent for the weld metal will differ to that of the parent metal. Welding processes such as FCAW will usually have 30% dilution of the parent metal into the weld pool and the amount of dilution can also be affected by other factors such as the type of joint, edge preparation and the intensity of welding current. A simple estimation of the value of carbon equivalent for the nominal composition of weld metal based on 30% dilution of the X80 steel for a FCAW process with flux-cored wire is approximately 0.32. This estimation is based on the composition of both parent steel and the consumable that are inserted into a spreadsheet. The spreadsheet can be found in the appendix. The results are plotted and can be seen from fig. X. 44 Carbon Equivalent, CEIIW, Value Based on Nominal Composition of Weld Metal 0.4 Carbon Equivalent, CEIIW 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 % Dilution of Parent Metal Fig. 26 Carbon Equivalent Value Based On The Nominal Composition Of Weld Metal With Respect To The Amount Of Dilution 3.7.6 Misalignment Linear misalignment between two plates butt welded together can cause stress concentration, contributing to high local stresses that is sufficient to induce hydrogen cracking either during welding, in service or both. Angular misalignment is equally harmful when there is a risk of hydrogen cracking. Angular misalignment can also reduce the degree of penetration of the weld. 45 4. Part 2 – Metallorgraphic Preparation 4.1 Cutting A weld specimen is required to undergo various stages of preparation prior to being examined under a microscope. First of all, a transverse section was removed from the weld and heat-affected zone. A saw was used to cut through the weld to produce the specimen. A filing tool was then used to produce a sufficiently flat surface on the specimen, eliminating the deep scratches created by the preceding sawing process. 4.2 Mounting When the transverse section of the weld has been obtained, it was mounted into thermosetting resin bakelite. This provides a larger area for better gripping during handling of the specimen. In addition, contact between the weld metal and bare hands was avoided; hence minimizing contamination and prevents the surface of the specimen from being scratched. 4.3 Grinding Grinding was performed to eliminate deep scratches on the surface of the specimen. The equipment used for wet grinding includes a flat grinding wheel, grade P120, P320, P500 and P1200 silicon carbide grinding papers and a source of clean water supply. The size of grit on the grinding paper decreases as the number specifying the grade of the paper increases, indicating that the P120 paper has the coarsest grit. The grinding process begins with grade P120 grinding paper, progressing to finer grades successively. When 46 grinding is first performed on the P120 paper, the specimen is orientated at 90 degrees between the filing scratches that were produced earlier on and the direction of the grind. Such a procedure ensures that the filing scratches have been properly grounded off from the surface. It is inappropriate for the initial grinding to be done in such a way that the direction of grind is same to the direction of the scratch lines left by the filing process. By doing so, it would be extremely difficult to differentiate between the new scratches created from grinding and the old scratches left from the filing process, thus making it impossible to determine if the former scratches are thoroughly removed. Subsequently, when all previous scratches have been completely ground off, the specimen is rotated by 90 degrees each time as the grinding process advances to finer grade grinding papers. After every grinding step, the specimen is rinsed to flush away any grit and ensure that no foreign particles are transferred to the finer grade paper. Wet grinding is performed by letting a continuous flow of water over the waterproof silicon carbide paper. The flowing water carries away particles that are dislodged during the grinding process. In addition, it acts as a lubricant and also as a coolant to dissipate any heat that is generated. It is important to provide adequate cooling as to avoid over heating the specimen during the grinding process to prevent changes to its microstructure by excess heat. 4.4 Polishing Polishing was performed in two stages, i.e., coarse polishing using a 3m diamond cloth of 250mm diameter and fine polishing using a 1m diamond cloth of 250mm diameter. Polishing cloths attached onto rotating wheels are the basic equipment required. After grinding was done on the finest grade grinding paper, the specimen was thoroughly rinsed before commencing the polishing process. Rubber gloves were worn to prevent contamination of the polishing cloth. A specific lubricant, which contains ethanol, was applied in order to wet the cloth. The specimen was held in circular motion on the cloth 47 with light pressure, in order to achieve a reasonably good polish. After coarse polishing, the specimen was rinsed with ethanol before being subjected to an ultrasonic cleaning treatment. The specimen was then rinsed again with ethanol before proceeding with fine polishing. At the completion of fine polishing, the specimen was again rinsed with ethanol and dried with a hair drier. At this stage, the surface of the specimen has a mirror like appearance. 4.5 Etching Before a specimen was etched, preparations such as proper cleaning and degreasing of the surface needs to be adequately performed. This is necessary in order to obtain a desirable etched specimen. Cleaning and degreasing was done by carefully applying a soap solution on the specimen and then rinsing it with a generous flow of tap water. Precaution is needed while washing the specimen as not to inflict any damage to the polished surface. Etching involves dipping the surface of the specimen into a suitable etching reagent. For a few seconds, the specimen was vigorously agitated to enable the etching reagent to react with the specimen effectively. Immediately, the specimen was then thoroughly washed under running water to remove any remaining etchant. The specimen was then rinsed with alcohol and dried using a hair drier. A visual inspection helps to check the extent of etching to the specimen. For a specimen that has been properly etched, a dull surface appears over the originally highly polished surface. However, getting a bright surface suggests that the etching process needs to be repeated. Special care should be taken as not to produce a badly etched specimen, resulting in badly stained or discoloured surface. An etched surface reveals visible microstructures. The etching reagent reacts with different grains to different extents and thus helps to reveal different types of crystals that 48 may be present, i.e. ferrite, acicular ferrite bainite or martensite and their boundaries. Fig. 10 shows the differences before and after etching. (i) (ii) Fig. 27 (I) Before Etching (Ii) After Etching In the polished state, the structure is hidden by the flowed layer – only a few polishing scratches are visible on the otherwise featureless surface Etching removes the flowed layer, thus revealing the crystal structure beneath 2% Nital is a common etching reagent for steel, while other reagents may be available for other metals and also to reveal different characteristics of a specimen. Segregation is visible on the specimen when it is etched with LePera’s reagent. This etching reagent is produced by mixing equal volumetric proportion of 4% of picric acid (picral) dissolved in methyl alcohol and 1% aqueous sodium metabisulphite. It reveals the solidification morphology that occurred from liquid to -ferrite by delineating microsegregation that is resulted from cellular and dendritic grain growth. Pale grey appearance is observed in areas of microsegregation. 49 4.6 Discussions Various stages of grinding and polishing in successions help to remove scratches and produce a smooth surface of the specimen. Furthermore, all surface contamination such as rust and dirt are removed. This makes it easy for the specimen to be observed without any imperfection that could corrupt the results. Etching with LePera’s reagent produces best results when the reagent is prepared immediately prior to etching. 50 5. Part 3 – Microscopy Analysis When the specimen has appropriately undergone metallographic preparation, it is ready to be analysed under both optical and electron microscopy. Microscopy analysis is essential for the specimen to be studied at high magnification levels. This helps to reveal all microscopic flaws such as cracks which are not visible when viewed with the naked eye. In addition, with the aid of etching, segregation of alloying elements would be visible and the microstructures of the weld specimen can be identified and examined. Understanding type and size of crystals in the microstructure could provide information on the mechanical properties of the weld. Subsequently, the susceptibility of the weld to HACC can be predicted and justified. 5.1 Optical Microscopy The reflected light microscope was used to check for cracks in the weld specimen. The equipment used is Olympus BX60m light microscope, on which the Olympus Dp11 digital camera was attached to. Images of the specimens were captured by the camera and transferred to a desktop computer for storage. Beginning from low magnification of 100x, the surface of the specimen was scanned to obtain an overall image of the formation of the weld. Once a crack was found, the region where the crack occurs was examined under higher magnification of 500x. The mode of cracking and the crack path were correlated to the influence of HACC in welds of low alloy steel. 5.2 Scanning Electron Microscopy The Philips XL20 scanning electron microscope was used to image the specimen. The scanning electron microscope is able to produce a high magnification view of the specimen that is unachievable by reflected light microscopy. The crack surfaces can be 51 examined for any inclusion and discontinuity that could initiate a crack. High magnification is vital for detection of fish eyes that are believed to be present in all fractures resulted from HACC. Fish eye is the formation of a pore with smooth shiny surface caused by entrapment of a non-oxidising gas, most possibly hydrogen, in the weld during solidification. As mentioned in previous chapters, atomic hydrogen is present in arc welding and highly soluble in the weld pool. Insufficient time for the hydrogen to diffuse causes it to concentrate within the weld, forming minute gas pores hence contribute to the formation of fish eyes. Discontinuity due to fish eye causes high stress concentration in the surrounding weld metal that is vulnerable for crack formation. 5.3 Electron Probe Micro-Analysis (EPMA) The specimen was then examined using electron probe micro-analysis using the Cameca SX51 Microprobe with four multicrystal Wavelength Dispersive Spectrometer (WDS) standardised using standards of pure elements. The surface of the specimen was mapped to produce an image of pattern of dispersion of elements within the weld. Dispersion of different elements can be identified as areas of different shades in the image. Heavier elements appear in darker regions while lighter elements appear in brighter regions. Areas of different shades reveal that the concentrations of elements are dependent on the solidification process. High concentrations of alloying elements are found in areas where segregation occurs. This is verified using the Energy Depressive X-ray Analyser (EDAX) facility. Determination of the composition of each element present in the weld is achieved by conducting line scans across the light and dark regions using EDAX. The images of mapped regions are digitally enhanced and colour is added to make the areas of segregation more apparent. As such better observation is possible and these images are showed in figs 28. 52 Fig 28. Mapped region in the weld The results obtained from the line scans are plotted in a graph. Segregations can be identified with the appearance of peaks in the graph. Peaks in Fig x. indicate areas with high concentrations of certain element as well as the amount present while horizontal dashed lines shows their background composition. It can be seen that in areas of microsegregation, there is a certain ratio between the peak composition and the background composition for each element. The data for plotting this graph is available in the appendix. 53 Weight % Composition Along Horizontal Line Within Weld 2 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 Position Wt% Si Wt% Mn Wt% Ni Fig 29. Line scan within the weld metal with the composition of certain elements 54 6. Results And Discussions 6.1 Weld Microstructure The microstructure of the FCAW weld at low heat input mainly consists of relatively coarse granular bainite together with ferrite laths. As heat input increases, the cooling time of the weld pool increases correspondingly and hence the bainitic microstructures becomes coarser while the ferrite laths increases in length. Non-metallic inclusions were also found scattered in low quantities within the weld metal. These inclusions are not uniformly sized and most appeared to be round in shape. They are believed to originate from the non-metallic materials of the flux in the consumable that are subsequently entrapped in the weld pool during the welding process. The presence of inclusions in the weld metal is not desirable as they have a detrimental effect on the weld. Inclusions cause discontinuities in the microstructure and this tends to weaken the weld and is known to be the point of initiation of fracture in welds. 6.2 Analysis Of Microsegregation And Macrosgregation 55 7. Conclusions There is not much conclusion that can be made at this stage of the report. This project in right on schedule and has been progressing at a satisfactory pace. Most of the equipment needed for the welding process has already been fabricated and experiment preparation is well done. In depth study of literatures in the field of HACC has been conducted and better understanding in this respective subject has been obtained. However more effort needs to be added to further improve this project, especially in understanding the welding processes involved and producing consistent specimens that can show the exact details which influence HACC. 56 8. Appendix 57 9. References 1. Althouse, A.D., Turnquist, C.H. and Bowditch, W.A., 1970. Modern Welding, The Goodheart_Willcox Co., Inc 2. Bailey et al, 1973. Welding steels without hydrogen cracking, Abington publishing 3. Bailey, N., 1994., Weldability of Ferritic Steels, Abington publishing 4. Henry, O.H. and Claussen, G.E., 1940. Welding Metallurgy, American Welding Society 5. Higgins, R.A., 1987. Materials For The Engineering Technician, Arnold, Hodder Headline Group 6. Jarmila Woodtli, 2000, Damage due to hydrogen embrittlement and stress corrosion cracking, Engineering Failure Analysis 7 (2000) 427±450 7. Lancaster, J.F., 1980. Metallurgy of Welding, George Allen & Unwin Ltd 8. M. Smialowski, 1962, Hydrogen in Steel, Perfamon Press 9. Steinberger, A. W. and J. Stoop, 1952, studies of the crack sensitivity of aircraft steels and weldments, Welding Journal 31, 527 58