The Uncertainty of Measurements
advertisement

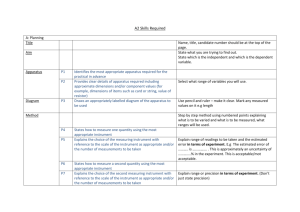
The Uncertainty of Measurements Adapted from UNC Guide, The University of North Carolina at Chapel Hill, Department of Physics and Astronomy http://www.physics.unc.edu/~deardorf/uncertainty/UNCguide.html Name: ___________________ Some numerical statements are exact: I have 2 brothers, and 1 + 2 = 3. However, whenever we need to measure something, there is suddenly a degree of uncertainty. And for any measurement to be complete, it should include an estimate of how certain you are about the measurement. Properly reporting an experimental result along with its uncertainty allows other people to make judgments about the quality of your work. When we make a measurement, we generally assume that some true value exists. The board does have some exact length. The object does have some exact mass. While we may never know this true value exactly, we can make measurements by different methods, or even make multiple measurements using the same method, to obtain a range of results. Then, we show the range of values that we believe includes the true value as: measurement = best estimate ± uncertainty For example, suppose you want to sell a gold necklace; you’ll want to know the mass in order to charge a fair market price. You know the mass is between 10 and 20 grams, but this is not a very precise estimate. Using an electronic balance, the necklace has a mass reading of 17.43 grams. This is more precise than the original estimate, but how do you know that it is accurate, and how confident are you that this measurement represents the true value of the gold’s mass? Since the digital display of the balance is limited to two decimal places, you could report the mass as m = 17.43 ± 0.01 g. Suppose you use the same electronic balance and obtain several more readings: 17.46 g, 17.42 g, 17.44 g. The average mass is now 17.44 ± 0.02 g. By now you may feel confident that you know the mass of this ring to the nearest hundredth of a gram, but how do you know that the true value definitely lies between 17.43 g and 17.45 g? You decide to use another balance and obtain a reading of 17.22 g. This value is clearly below the range of values found on the first balance, and under normal circumstances, you might not care, but you want to be fair. So what do you do now? To help answer these questions, we should first define the terms accuracy and precision: Accuracy is how close your measured value is to the true or accepted value. Measurement error is the amount of inaccuracy. Precision is a measure of how well a result can be determined using your resources. It is the also the reliability or reproducibility of the result. The statement of uncertainty associated with a measurement should include factors that affect both the accuracy and precision of the measurement. Caution: Unfortunately the terms error and uncertainty are often used interchangeably to describe both imprecision and inaccuracy. This usage is so common that it is impossible to avoid entirely. Whenever you encounter these terms, make sure you understand whether they refer to accuracy or precision, or both. When analyzing experimental data, it is important that you understand the difference between precision and accuracy. Precision indicates the quality of the measurement, without any guarantee that the measurement is “correct.” Accuracy, on the other hand, assumes that there is an ideal value, and tells how far your answer is from that ideal, “right” answer. These concepts are directly related to random and systematic measurement errors. Types of Errors Measurement errors may be classified as either random or systematic, depending on how the measurement was obtained (an instrument could cause a random error in one situation and a systematic error in another). Random errors are statistical fluctuations (in either direction) in the measured data due to the precision limitations of the measurement device. Random errors can be evaluated through statistical analysis and can be reduced by averaging over a large number of observations (see standard error). Systematic errors are reproducible inaccuracies that are consistently in the same direction. These errors are difficult to detect and cannot be analyzed statistically. Unlike random errors, systematic errors cannot be detected or reduced by increasing the number of observations. Our strategy is to reduce as many sources of error as we can, and then to keep track of those errors that we can’t eliminate. It is useful to study the types of errors that may occur, so that we may recognize them when they arise. Common sources of error in physics laboratory experiments: Incomplete definition (may be systematic or random) - One reason that it is impossible to make exact measurements is that the measurement is not always clearly defined. For example, if two different people measure the length of the same rope, they would probably get different results because each person may stretch the rope with a different tension. The best way to minimize definition errors is to carefully consider and specify the conditions that could affect the measurement. Failure to account for a factor (usually systematic) – The most challenging part of designing an experiment is trying to control or account for all possible factors except the one independent variable that is being analyzed. For instance, you may inadvertently ignore air resistance when measuring free-fall acceleration, or you may fail to account for the effect of the Earth’s magnetic field when measuring the field of a small magnet. The best way to account for these sources of error is to brainstorm with your peers about all the factors that could possibly affect your result. This brainstorm should be done before beginning the experiment so that arrangements can be made to account for the confounding factors before taking data. Sometimes a correction can be applied to a result after taking data to account for an error that was not detected. Environmental factors (systematic or random) - Be aware of errors introduced by your immediate working environment. You may need to take account for or protect your experiment from vibrations, drafts, changes in temperature, electronic noise or other effects from nearby apparatus. Instrument resolution (random) - All instruments have finite precision that limits the ability to resolve small measurement differences. For instance, a meter stick cannot distinguish distances to a precision much better than about half of its smallest scale division (0.5 mm in this case). Failure to calibrate or check zero of instrument (systematic) - Whenever possible, the calibration of an instrument should be checked before taking data. When making a measurement with a balance or spring scale, always check the zero reading first. Re-zero the instrument. It is a good idea to check the zero reading throughout the experiment. Physical variations (random) - It is always wise to obtain multiple measurements over the entire range being investigated. Doing so often reveals variations that might otherwise go undetected. These variations may call for closer examination, or they may be combined to find an average value. Parallax (systematic or random) - This error can occur whenever there is some distance between the measuring scale and the indicator used to obtain a measurement. If the observer's eye is not squarely aligned with the pointer and scale, the reading may be too high or low. When we use video cameras to capture motion, we may experience this. Instrument drift (systematic) - Most electronic instruments have readings that drift over time. The amount of drift is generally not a concern, but occasionally this source of error can be significant and should be considered. Lag time and hysteresis (systematic) - Some measuring devices require time to reach equilibrium, and taking a measurement before the instrument is stable will result in a measurement that is generally too low. The most common example is taking temperature readings with a thermometer that has not reached thermal equilibrium with its environment. A similar effect is hysteresis where the instrument readings lag behind and appear to have a “memory” effect as data are taken sequentially moving up or down through a range of values. Hysteresis is most commonly associated with materials that become magnetized when a changing magnetic field is applied. Personal errors come from carelessness, poor technique, or bias on the part of the experimenter. The experimenter may measure incorrectly, or may use poor technique in taking a measurement, or may introduce a bias into measurements by expecting (and inadvertently forcing) the results to agree with the expected outcome. Gross personal errors, sometimes called mistakes or blunders, should be avoided and corrected if discovered. As a rule, gross personal errors are excluded from the error analysis discussion because it is generally assumed that the experimental result was obtained by following correct procedures. The term human error should also be avoided in error analysis discussions because it is too general to be useful. Estimating Experimental Uncertainty for a Single Measurement Any measurement you make will have some uncertainty associated with it, no matter how precise your measuring tool. How do you actually determine the uncertainty, and once you know it, how do you report it? The uncertainty of a single measurement is limited by the precision and accuracy of the measuring instrument, along with any other factors that might affect the ability of the experimenter to make the measurement. In this exercise you will measure as accurately as possible the size of an index car, the area of the floor of the room and the volume of a tennis ball. You will record your measurement and estimate the precision of each measurement. The precision will depend on how fine is the scale of your measuring instrument. As an example, you might have measured the index card to be 12.35 cm. However, given the scale of the ruler, you can only be accurate to the nearest half millimeter. This would be recorded as 12.35 cm ± 0.05 cm. That means the actual length falls between 12.30 cm and 12.40 cm. However, when you try to measure the diameter of a tennis ball, the uncertainty might be ± 5 mm, because of parallax and the ambiguity in the definition of the tennis ball’s diameter (it’s fuzzy!). In both of these cases, the uncertainty is greater than the smallest divisions marked on the measuring tool (1 mm). Unfortunately, there is no general rule for determining the uncertainty in all measurements. Since you made the measurement, you can best evaluate and quantify the uncertainty of the measurement based on all the possible factors that affect the result. Data (include uncertainty for each measurement) A. Index Card Data Length Width Length Width Trial 1 Trail 2 Trial 3 Trial 4 Average B. Room Data Trial 1 Trail 2 Trial 3 Trial 4 Average C. Tennis Ball Diameter Trial 1 Trail 2 Trial 3 Trial 4 Average Calculations (show all work) A. Find the best estimate (the mean or average) of each measurement. Be sure to include the uncertainty in the average. B. Calculate the area of the index card. Example: If one side was 12.35 cm ± 0.05 cm and another was 7.30 cm ± 0.05 cm, the best estimate of the area is 12.35 cm x 7.30 cm, or 90.155 cm2. C. Use significant figures to find the appropriate answer. For example, while 12.35 cm has four meaningful digits or significant figures, 7.30 cm has only three significant figures, so it has a greater uncertainty and this limits the precision of the calculated area. So even though the calculated area of 90.155 cm 2 looks like it has five significant figures, only the first three have any physical meaning, or significance. So the answer that expresses the precision of the measurements must also have three significant figures. Thus, the appropriate answer is 90.2 cm2 D. Find the range of possible areas and express the area as your best estimate ± the calculated error. a. The smallest possible area is calculated from the lowest values of the ranges in lengths. This would be the product of 12.30 cm (12.35 - 0.05) and 7.25 cm (7.30 - 0.05), which equals 89.175 cm2. Once again, 7.25 cm has only three significant figures, so it has the greater fractional error and, hence, is the limiting factor in the precision of the calculated area. So the appropriate answer for the lower range of possible areas is 89.2 cm2. b. Likewise, the largest possible area is calculated from the highest values of the ranges in lengths. This would be the product of 12.40 cm (12.35 + 0.05) and 7.35 cm (7.30 +0.05), which equals 91.15 cm 2. Again, the appropriate answer must have three significant figures so 91.15 cm2 is rounded to 91.2 cm2. E. So, although the best estimate of the area is 90.2 cm2, the actual area falls within the range of 89.2 to 91.2 cm2. The experimentally determined area can be expressed as 90.2 ± 1.0 cm2. The value 1.0 cm2 is called the uncertainty of the area and reflects the uncertainties inherent in any measurement. F. Calculate the area of the room and the uncertainty. 3 G. Calculate the volume of the tennis ball and the uncertainty (remember d=2r and use the formula V 4r ). 3 Conclusions A. Record the results of three other people in the following table. Name: Index Card Area Room Area Tennis Ball volume B. Do the ranges of your data overlap theirs? Why? Why not? C. What are some possible errors that could have occurred?