Error and Uncertainty Notes
advertisement

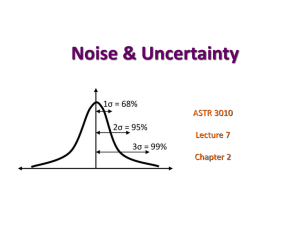
Errors and Uncertainty In science, every measurement will have limitations on its accuracy. To indicate how well a data point should be represented, significant figures and uncertainties are used. The history of science has many disastrous examples of where uncertainties in measurements were not taken into account WHY DO WE HAVE UNCERTAINTY ? 1. Measuring Uncertainty – the lowest increment on the measuring device determines the precision of the instrument. You cannot have a precision greater than the last digit marked on the instrument!!! - ruler marked every mm d = 45.6 cm 0.1 cm -thermometer marked every o T = 12 oC 1 oC Note that uncertainty is limited to the last place setting. It is incorrect to write l = 18.5 cm 1.5 cm. 2. Random Errors – Many experiments, when repeated, will yield slightly different results each time. Random errors are produced by unknown and unpredictable variations in the experimental situation. Ex – when shooting bullets, it is unlikely that they have the same exit speed due to variations in the cartridge, fluctuations in gunpowder explosion etc… Ex – when bouncing a ball, it is unlikely to rebound to the same height each time bounce is repeated. This may be due to orientation of ball in air, orientation of ball as it hits surface, uneven surface etc… A simple technique to deal with random errors is to repeat the experiment many times. Find the mean of the data values. Find the variation in the data by taking Variation = high - low 2 - data would be represented by mean variation Other sophisticated methods are used such as working out the standard deviation but we will get into these methods as needed. Small random errors means experiment has high precision. 3. Systematic Errors – are errors associated with a particular instrument or experimental technique. Systematic errors are not reduced by repeating measurements. -is measuring device perfectly accurate ( calibration)? - is device zeroed ? -is the experiment being performed in a wind or presence of air currents? -is there a bias on behalf of the experimenter? ( right handed/left handed) -is odometer on car accurate if tires have been changed ? most electrical equipment is assumed to have a 5% systematic error Small systematic errors means experiment has high accuracy The Mathematics of Uncertainty Data value Absolute Uncertainty Relative Uncertainty x x x x Example: Height: 16.8 cm Absolute Uncertainty : 0.5 cm Note that absolute error has same units as measurement Relative Uncertainty : Relative Uncertainty has no units Note that relative uncertainty can be improved by measuring over a longer time period or greater length. 0 . 5 cm 16 . 8 cm 0 . 03 3 % Addition / Subtraction of Measurements When two measurements are combined, the uncertainty of each should first be expressed in absolute form. The absolute uncertainty of the sum/difference is the sum of the absolute uncertainties in the two numbers. Ex: ( 4 m ± 1m) + ( 12 m ± 2 m) = 16 m ± 3 m Ex: ( 952 ± 6) kg - ( 554 ± 10.) kg = ( 398 ± 16) kg Multiplication / Division Numbers should first be expressed as relative uncertainty The relative uncertainty in the result is the sum of the individual relative uncertainty. Ex: ( 4.0 ± 1.0) mm * ( 12 ± 2) mm ( 4.0mm ± 0.25) * ( 12 mm ± 0.2) ( 48 mm2 ± 0.4 ) or ( 48 mm2 ± 40% )