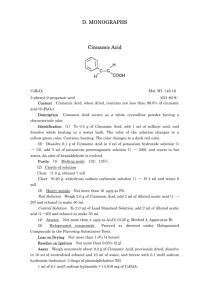
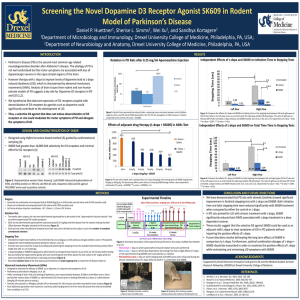
Analyzing the graphs suggest that cinnamic acid is a noncompetitive
advertisement

Analyzing the graphs suggest that cinnamic acid is a noncompetitive inhibiter of tyrosinase’s reaction with L-Dopa. When graphed out, both the slope and y-intercept of the inhibited data were roughly double that of the uninhibited results. The x-intercept should be the same if it is noncompetitive, however there is a difference between the two when using the line equations and setting y=0. The x-int for inhibited was -2481, while uninhibited it was -3764. Despite this difference, noncompetitive seemed to fit the data best. A quick search of cinnamic acid and tyrosinase reveals several previous experiments which have determined that it is indeed noncompetitive. A link to a French study is provided below, where the Ki was found to be 1.994 (MUCH higher than our results). When comparing the Km values found amongst the class, there was a clear difference between the L and D Dopa values. L-Dopa averaged to .1876 mM while D-Dopa averaged to 1.359 mM. This indicated a clear preference for the L-Dopa stereoisomer, as lower Km values means the enzyme and substrate bind together more tightly. http://cat.inist.fr/?aModele=afficheN&cpsidt=16745827