The mole Concept
advertisement

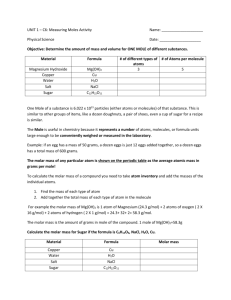
The mole Concept Background: John Dalton- Assigned an arbitrary mass to each element. - The mass of hydrogen is 1 (lightest element). - Carbon was 12, since it was 12 times more massive than hydrogen. - Calculated relative masses for several different elements. Gay-Lussac: - Performed experiments with gases at standard temperature and pressure. Example of his experiment: 2 L of hydrogen reacted with 1 L of oxygen to form 2 L of water vapour. 2 L of CO reacted with 1 L of oxygen gas to form 2 L of CO2 gas. Amadeo Avogadro: - Used Gay-Lussac’s result to come up with Avogadro’s Hypothesis. - Equal volume of different gases, at the same temperature and pressure, contain the same number of particles. - Therefore 1L of Oxygen has the same number of oxygen molecules as 1L of nitrogen molecules (@ STP). To measure the mass of elements, it was decided that the mass of 1 atom of Cabon-12 isotope was going to be 12 atomic mass unit (a.m.u.). Every other element was then measured relative to carbon-12. This way, the mass of hydrogen = 1 a.m.u Helium = 2.0 a.m.u Using all this information, we can now introduce a very important concept in chemistry. (you can start copying) The mole The mass indicated in the periodic table is the average mass of all the different isotopes of that particular element. The mass of one atom of Carbon is 12.0 u. The mass of one atom of Lithium is 6.9 u. The mass shown in the periodic table can be translated into a practical unit by introducing the concept of THE MOLE! A mole is the number of carbon atoms present in exactly 12 g of carbon (Carbon-12 isotope). 1 mole = 6.02 x 10 23 In use, the mass of one molecule of a substance is measured in A.M.U.'s while a mole (6.02 x 10 23 atoms) of a substance is measured in grams. We utilise the periodic table to get our masses. Example The mass of one atom of sodium is 23.0 A.M.U.'s The mass of one mole of sodium is 23.0 grams. NOTE : When using the atomic mass on the periodic table, round off the number given to one (two for AP chem) decimal place. Example - What is the mass of one mole of copper atoms ? - 63.55 grams Determine the mass of 1 mole ( or commonly known as MOLAR MASS): a) NaCl 23.0 + 35.5 = 58.5 grams B) potassium dichromate - K2Cr2O7 K 2 x 39.1 = 78.2 Cr 2 x 52.0 = 104.0 O 7 x 16.0 = 112.0 ----294.2 grams The proper units for molar mass are grams per mole or g/mol Example - What is the molar mass of aluminum carbonate ? - Al2(CO3)3 Al 3 x 27.0 = 81.0 C 3 x 12.0 = 36.0 O 9 x 16.0 = 144.0 ----251.0 g/mol What is the malar mass of fluorine gas? (F2 = 2 x 19.00 g) What is the molar mass of neon gas? Assignment: - Complete mole worksheet #1