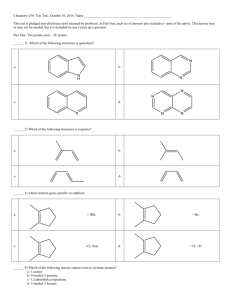
CHEM 101 – EXAM 4 – PRACTICE PROBLEMS
advertisement

CHEM 101 – EXAM 3 – PRACTICE PROBLEMS 1. Of the following choices: C+2, Na+3, Si-4, and Zn-1 a) Which would have the lowest first ionization energy? b) Which would have the highest second ionization energy? 2. Which has the largest atomic radius and why: a) Li, Na, K, or Rb b) N-2 or B-4 3. Which ion in problem 1 is least likely to gain an electron? 4. a) Is NaCl ionic or covalent? b) Does it have a high or low boiling point? c) Draw its Lewis structure. 5. Which has a more stable bond: N2 or O2? 6. a) b) c) d) e) f) Draw the Lewis structure for NO3-. Draw any resonance structures for it. Calculate the formal charges on each atom. What is its molecular geometry? Does it have a dipole moment? What are the bond angles in the structure? 7. a) What is the hybridization of the central atom in AsH3? b) What is the molecular geometry of AsH3? c) What are the bond angles present in AsH3? 8. Calculate ∆H using bond dissociation energies in the following reaction: (all are gases) CH4 + 2 Cl2 + F2 → CF2Cl2 + 2 HF + 2 HCl The structure of CF2Cl2 has C in the center with 2 F’s and 2 Cl’s surrounding it. 9. How many sigma and pi bonds are in CH3-CH-(CH3)-CH=CH-C≡CH? 10. a) What is the geometry of AH3 where A is any group 5 element? b) What are the bond angles? 11. a) Does H2O have a dipole moment? b) How many individual bond moments does it have? 12. a) b) c) d) What is the molecular orbital diagram of Li2? What is its bond order? Is it paramagnetic or diamagnetic? What is its electron configuration?