chm 103 general chemistry
advertisement
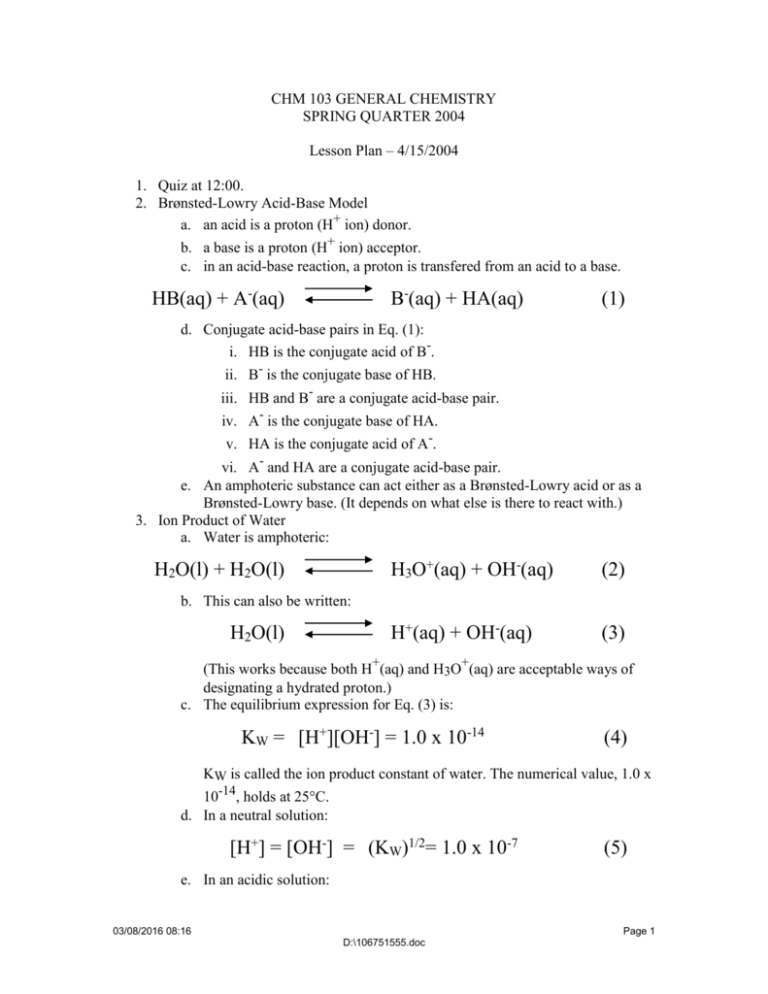
CHM 103 GENERAL CHEMISTRY SPRING QUARTER 2004 Lesson Plan – 4/15/2004 1. Quiz at 12:00. 2. Brønsted-Lowry Acid-Base Model a. an acid is a proton (H+ ion) donor. b. a base is a proton (H+ ion) acceptor. c. in an acid-base reaction, a proton is transfered from an acid to a base. HB(aq) + A-(aq) B-(aq) + HA(aq) (1) d. Conjugate acid-base pairs in Eq. (1): i. HB is the conjugate acid of B-. ii. B- is the conjugate base of HB. iii. HB and B- are a conjugate acid-base pair. iv. A- is the conjugate base of HA. v. HA is the conjugate acid of A-. vi. A- and HA are a conjugate acid-base pair. e. An amphoteric substance can act either as a Brønsted-Lowry acid or as a Brønsted-Lowry base. (It depends on what else is there to react with.) 3. Ion Product of Water a. Water is amphoteric: H2O(l) + H2O(l) H3O+(aq) + OH-(aq) (2) H+(aq) + OH-(aq) (3) b. This can also be written: H2O(l) (This works because both H+(aq) and H3O+(aq) are acceptable ways of designating a hydrated proton.) c. The equilibrium expression for Eq. (3) is: KW = [H+][OH-] = 1.0 x 10-14 (4) KW is called the ion product constant of water. The numerical value, 1.0 x 10-14, holds at 25°C. d. In a neutral solution: [H+] = [OH-] = (KW)1/2= 1.0 x 10-7 (5) e. In an acidic solution: 03/08/2016 08:16 Page 1 D:\106751555.doc [H+] > [OH-] , (6) [H+] > 1.0 x 10-7 (7) [OH-] < 1.0 x 10-7 (8) f. In a basic solution: [H+] < [OH-] , (9) [H+] < 1.0 x 10-7 (10) [OH-] > 1.0 x 10-7 (11) 4. pH and pOH a. Definition of pH: pH = -log10[H+] (12) [H+] = 10-pH (13) b. Definition of pOH: pOH = -log10[OH+] (14) [OH-] = 10-pOH (15) c. In a neutral solution: pH = pOH = 7 (16) d. In an acidic solution: pH < 7 (17) pOH > 7 (18) e. In a basic solution: pH > 7 03/08/2016 08:16 (19) Page 2 D:\106751555.doc pOH < 7 (20) f. In any (aqueous) solution: pH + pOH = 14 (21) 5. pH of Strong Acids a. A strong acid is strong by virtue of being totally ionized in aqueous solution. For example, a 0.1 M solution of HCl is 0.1 M in H+ and in Cl-. Thus: [H+] = 1.0 x 10-1 (22) pH = - log10(10-1) = 1.0 (23) And a 0.05 M solution of HNO3 has: [H+] = 5.0 x 10-2 (24) pH = - log10(5.0 x 10-2) = - log10(5.0) - log10(10-2) = -0.7 + 2.0 = 1.3 (25) 6. pH of Weak Acids a. A weak acid is weak by virtue of being incompletely ionized in aqueous solution. Weak acids can be either: i. Molecules containing an ionizable hydrogen atom. HA(aq) + H2O(l) HA(aq) H3O+(aq) + A-(aq) (26a) H+(aq) + A-(aq) (26b) (Eq. (26a) follows the Brønsted-Lowry Acid-Base model, showing proton transfer from HA to H2O, while Eq. (26b) is the more conventional representation of the ionization of a weak acid.) ii. Ions containing an ionizable hydrogen atom. NH4+(aq) + H2O(aq) NH4+(aq) H3O+(aq) + NH3(aq) (27a) H+(aq) + NH3(aq) (27b) 03/08/2016 08:16 Page 3 D:\106751555.doc (Eq. (27a) follows the Brønsted-Lowry Acid-Base model, showing proton transfer from NH4+ to H2O, while Eq. (27b) is the more conventional representation of the ionization of a cation acting as a weak acid.) 7. Equilibrium Constant for the Dissociation of a Weak Acid a. We can write the reaction for the dissociation of acetic acid (HC2H3O2) as: H+(aq) + C2H3O2-(aq) HC2H3O2(aq) (28) And we can write the equilibrium expression for Eq. (28) as: K28 = [H+][C2H3O2-] [HC2H3O2] = 1.8 x 10-5 (29) Suppose we have a 0.05 M aqueous solution of acetic acid, and we want to know [H+] and pH for the solution. We can set up an equilibrium table: HC2H3O2 H+ C2H3O2- [ ]0 0.05 0 0 [ ]eq 0.05 - x x x = 1.8 x 10-5 (30) Plugging numbers into Eq. (29) we get: x2 0.05 -x We “guess” that x << 0.05 and simplify Eq. (30) to get: x2 0.05 = 1.8 x 10-5 (30) x2 = 0.05 x 1.8 x 10-5 (31) x2 = 0.9 x 10-6 (32) x= 0.3 x 10-3 03/08/2016 08:16 Page 4 D:\106751555.doc Now we can add the final line to the equilibrium table: HC2H3O2 H+ C2H3O2- [ ]0 0.05 0 0 [ ]eq 0.05 - x x x [ ]eq 0.05 - 0.0003 3.0 x 10-4 3.0 x 10-4 [ ]eq 0.05 3.0 x 10-4 3.0 x 10-4 Now we can check our approximation that 0.05 - x = 0.05. We found that x was 0.0003, which would make 0.05 - x = 0.0497. But in fact, x = 0.05 is close enough. 03/08/2016 08:16 Page 5 D:\106751555.doc