Chemical Reactions
advertisement

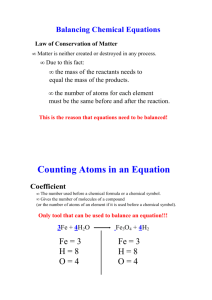
Chemical Reactions Content • • • • • • Reaction Review Writing and Balancing Chemical Equations Types of Reactions Predicting Products Predicting States of Matter Nature of Reactions Content • • • • • • • Reaction Review Balancing Chemical Equations Writing Chemical Equations Types of Reactions Predicting Products Predicting States of Matter Nature of Reactions 3 Types of Compounds Ionic Metal + Non-Metal Cation (+) + Anion (-) Covalent / Molecular Non-Metal + Non-Metal Prefixes Acid + H + Anion Chemical Reactions have two parts…. Reactants CH4 + O2 Reactants Products CO2 + H2O Products The order you list the reactants and products never matters Chemical: Involves a change in chemical composition (atoms are rearranged). Signs include: •Heat, Light, Sound (energy released/absorbed) •Formation of a new gas (bubbles, fizzes) •Formation of a precipitate Mass is neither lost nor gained (Law of Conservation of Mass) for both changes. Chemical change Physical changes Content • Reaction Review • Writing and Balancing Chemical Equations Chapter 8 pages 267 - 289 • Types of Reactions • Predicting Products • Predicting States of Matter • Nature of Reactions HONClBrIF The Missing Link HONClBrIF: All of these elements exist as diatomic molecules (not single atoms) in nature: H2 O2 N2 Cl2 Br2 I2 F2 A group of diatomic molecules 3 Requirements of a Chemical Equation • The equation must represent all the facts • All chemical formulas must be written correctly – Charges must add to zero – Cation, followed by anion – Diatomic elements written as diatomics • The Law of Conservation of Matter applies – Equation must balance Tips to Balancing Equations 1. Write correct skeletal equation 2. Balance the equation so each side has the same number of atoms of each element 3. Balance using coefficients, never change subscripts ex: __Fe + __O2 __Fe2O3 Tips to Balancing Equations 4. Coefficients apply to all the elements in a compound ex. 3C4H10 CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 12 C CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 30 H 12 C CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 2Ca(NO3)2 30 H 12 C CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 12 C 30 H 2Ca(NO3)2 2 Ca CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 12 C 30 H 2Ca(NO3)2 2 Ca 4N CH4 + 2O2 CO2 + 2H2O Coefficients indicate the number of each substance. To find the # of atoms, multiply the coefficient by subscripts. 3C4H10 12 C 30 H 2Ca(NO3)2 12 O (2x3x2) 2 Ca 4N Tips to Balancing Equations 5. O’s and H’s should be balanced last 6. Balance polyatomic ions as units (don’t count the atoms inside). 7. When you’re having trouble, put a 2 as the first coefficient and try again (then 3, then 4, …) Example: __Al + __I 2 3 2 2 __AlI 3 Examples: 6 2 __P4 + __Cl 2 __ CaCl2 + __NaNO 3 4 __PCl 3 2 __Ca(NO3)2 + __NaCl Example: __C4H10 + __O2 __CO2 + __H2O Example: ? 2 __C4H10 + __O __CO 4 5 2O 2 + __H 13 O Example: 2 13 __C H + __O 4 10 2 8 10 __CO + __H O 2 2 Example: Hydrogen + Oxygen Water 2 2 __H 2 2O + __O2 __H Example: Aluminum + Oxygen Aluminum Oxide 4 __Al 2 2O3 3 2 __Al + __O