equations atoms
advertisement

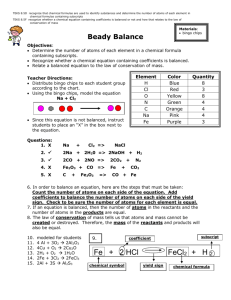
Daily Lesson Information Day/Dates : Lesson Focus: Lesson Objectives: Balancing Equations practice SWBAT: balance a chemical equation using pictures SWBAT: balance a chemical equation using multiplication of atoms (Performance Descriptors) Students will be able to: ILS (Illinois Learning Standards) CRS (College Readiness Standards) Reading Strategies Materials: Word of the Day Bell Work: Inquiry 12.C.4b Analyze and explain the atomic and nuclear structure of matter. Understand basic scientific terminology Translate information into a table, graph, or diagram Computer w/internet balance Engage Explore Explain Elaborate Evaluate (Circle all that apply) Before: Students will be asked to draw a picture representing a given chemical equation. Instructional Activities: Detailed narrative (description) of ALL instructional strategies and tools. During: Using the online simulation, students will have to add more molecules in order to make the equation balanced. Students can check their work by counting up the atoms to make sure they add up correctly and when they receive the smiley face. After: Students will be given the opportunity to balance ever increasingly difficult equations. They will record their score as they move through the 3 levels. Modifications: Assessment: Measuring tools to assess student mastery of lesson’s objectives. Homework: Counting atoms in a picture. Using a balance diagram. Using a bar graph. Practice problem score. Self evaluation. Name_____________________________________ Date______________ Period _______________ Balancing Chemical Reactions PhET Simulation Go to the following website. http://tinyurl.com/63yma4y Click on the button. tab, click on the button labeled “Bar Charts”. On the Review 1. The reactants in a chemical reaction are located to the (left of the arrow / right of the arrow). 2. The small numbers written after the element symbol are called (subscripts / coefficients). 3. The big numbers written before the molecule are known as (subscripts / coefficients). 4. If no number is written as the subscript or coefficient, then a number of _______ is implied. 5. Why do we balance chemical reactions? ______________________________________________ __________________________________________________________________________________ Activity You will be balancing each chemical reaction. Click on the button labeled “Make Ammonia”. Change each of the coefficients for each molecule balance the reaction. You will know it is balanced when you see the . Go through the 3 reactions and fill in the information below once it is balanced. Make Ammonia ______ N2 + ______ H2 _______ NH3 How many molecules are there for each chemical? How many atoms are there for each element on each side of the arrow? N= Separate Water ______ H2O ______ H2 + ______ O2 H= N= H= How many molecules are there for each chemical? How many atoms are there for each element on each side of the arrow? H= O= H= O= ______ CH4 + ______ O2 ______ H2O + ______ CO2 Combust Methane How many molecules are there for each chemical? How many atoms are there for each element on each side of the arrow? C= H H= O= C= H= O= Summary for Balancing Reactions 1. Do you need the same number of each element in a reaction? ________ Explain how you know. 2. The coefficients you filled in are the same as the number of (atoms / molecules). 3. How would you mathematically determine the number of atoms for each element using the balanced chemical reaction? 4. When you balance reactions, you add coefficients. Why can’t you add or change the subscripts in the chemical formula? 5. What is the smallest coefficient you can have when balancing? ________ Why would you not want to use a coefficient of 0 to balance any reaction? __________________________________________________________________________________ Balancing Game Click on the tab. Start on level 1. Leave the timer on. Record your data below. Score 1st Attempt Level 1 Level 2 Level 3 2nd Attempt Best Time