Counting Atoms in an Equation
advertisement
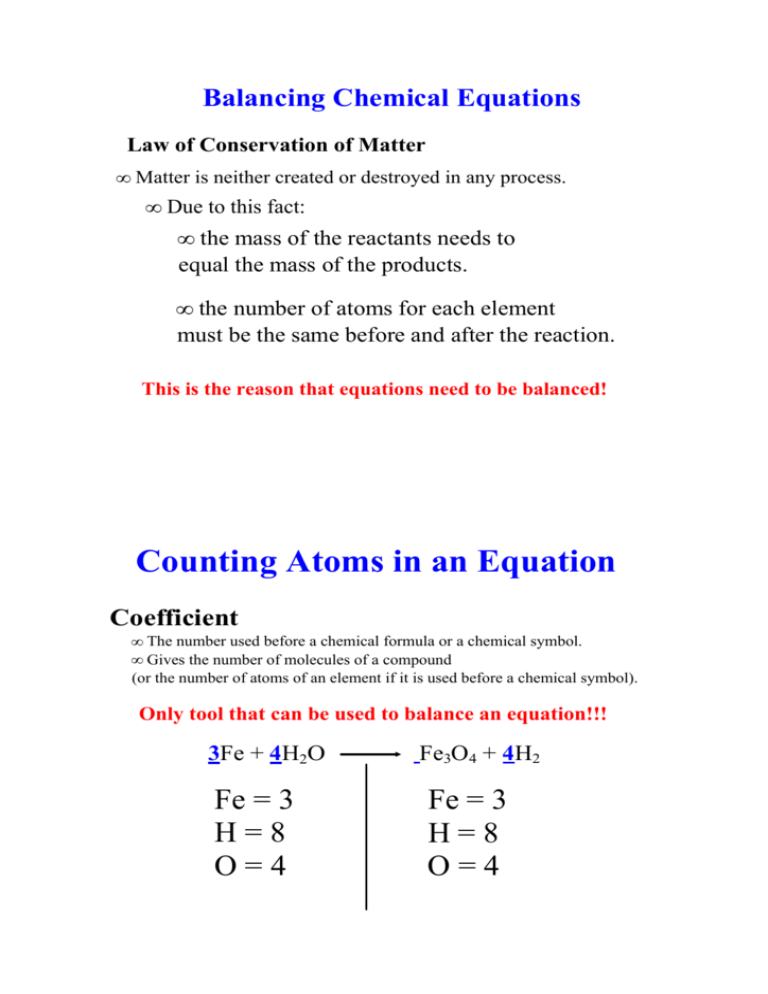
Balancing Chemical Equations Law of Conservation of Matter • Matter is neither created or destroyed in any process. • Due to this fact: • the mass of the reactants needs to equal the mass of the products. • the number of atoms for each element must be the same before and after the reaction. This is the reason that equations need to be balanced! Counting Atoms in an Equation Coefficient • The number used before a chemical formula or a chemical symbol. • Gives the number of molecules of a compound (or the number of atoms of an element if it is used before a chemical symbol). Only tool that can be used to balance an equation!!! 3Fe + 4H2O Fe3O4 + 4H2 Fe = 3 H = 8 O = 4 Fe = 3 H = 8 O = 4 3Fe + 4H2O Fe3O4 + 4H2 Fe = 3 H = 8 O = 4 Fe = 3 H = 8 O = 4 Notice: 1. The symbols are written in the same order on both side of the equation. 2. The subscripts are multiplied by the coefficients. 3. If there is no coefficient or subscripts it is assumed to be 1. When the left side matches the right side the equation is balanced! Balanced versus Unbalanced Try to classify the balanced and unbalance equations below: Balanced Unbalanced 2K + MgBr2 2KBr + Mg KClO3 KCl + O2 SiCl4 Si + 2Cl S8 + O2 SO3 2Ag2O Ag + O2 CH4 + 2O2 CO2 + 2H2O Steps to Balance a Chemical Equation Overview 1. Write out the equation (usually this is done for you). 2. Count the number of atoms on each side of the equation. 3. Add coefficients to balance the numbers of atoms on each side of the equation. 4. Write out the balanced equation with the correct coefficients. 5. This is an optional step ­ Recount the atoms to check your work. Steps 1 and 2 1. Write out the equation (usually this is done for you). 2. Count the number of atoms on each side of the equation. H2 + O2 H2O No ed c lan a t B H = 2 O = 2 H = 2 O = 1 Step 3 3. Add coefficients to balance the numbers of atoms on each side of the equation. You can put a coefficient before any "player" in an equation. H2 + O2 H2O H = 2 O = 1 H = 2 O = 2 Step 4 4. Write out the balanced equation with the correct coefficients. 2H2 + O2 2H2O 5. This is an optional step ­ Recount the atoms to check your work. 2H2 + O2 2H2O d lan Ba a ced nd e eck h C H = 4 O = 2 H = 4 O = 2