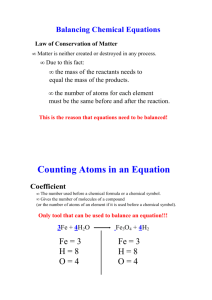
To balance an equation we assign arbitrary coefficients and solve

To balance an equation we assign arbitrary coefficient to each compound that participates in the reaction, and solve the system of equations that result from it. We obtain an equation for the coefficients from each individual element.
In our case we write: a C
2
H
6
+ b O
2
→ d CO
2
+ e H
2
O
So now we balance the amount of elements atoms on each side of the reaction equation:
For carbon, on the left hand side we have 2 a atoms while on the right hand side we have d carbon atoms. Hence:
2 a = d
For hydrogen, on the left we have 6 a hydrogen atoms while on the right we have 2 e hydrogen atoms. Therefore:
6 a =2 e
Or (dividing by 2):
3 a = e
And now we ar left with the equation for the oxygen. On the left hand side we have 2 b oxygen atoms while on the right we have 2 d + e oxygen atoms. So the equation is:
2 b =2 d + e
So far we obtained three equations for the coefficients:
2 a
3 a
2 b
d e
2 d
e
Since we have four unknowns ( a,b,d & e ) and only three equations, the system has infinite number of solutions. In this case we can assign one unknown an arbitrary value and calculate the rest of the unknown coefficients accordingly.
In our case it is most convenient to set a value for a , and we will set it as a =1.
Then we get: d
2 e
3
2 b
7
b
7
2
Therefore a valid balanced equation is: a C
2
H
6
+ b O
2
→ d CO
2
+ e H
2
O
7
C
2
H
6
+
2
O
2
→2CO
2
+3H
2
O
However, since it is customary to have only integers as coefficients, we have to multiply all the coefficients by 2 (to keep the equation balanced):
2C
2
H
6
+7O
2
→4CO
2
+6H
2
O
Note: If some of the compounds in the reaction end up with fractional coefficients, just multiply the equation by the least common denominator of the fractions.