NOMENCLATURE – HYDROCARBONS
advertisement

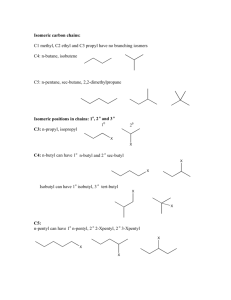
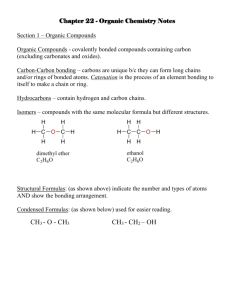
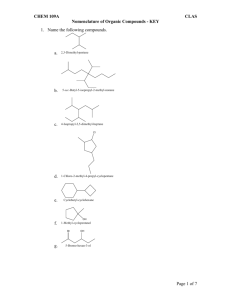
Laura B. Sonnichsen Chemistry 203 NOMENCLATURE – HYDROCARBONS 1. The number of carbons is indicated by the prefix: C1 C2 C3 C4 C5 2. Meth Eth Prop But Pent C6 C7 C8 C9 C10 Hex Hept Oct Non Dec The suffix indicates the class of compound: ane: alkanes ene: alkenes yne: alkynes 3. Alkyl groups are formally derived from the corresponding alkane by removal of one hydrogen (CnH2n+1). [Nomenclature – replace “e” with “yl”] CH3-CH3 CH3-CH2-CH3 4. ethane propane CH3-CH2ethyl group CH3-CH2-CH2- propyl group Common Names: (Hydrocarbons) Name Structure Alkyl Group(s) Name Abbreviation Methane CH4 CH3 Methyl Me Ethane CH3CH3 CH3CH2 Ethyl Et Propane CH3CH2CH3 CH3CH2CH2 n-Propyl n-Pr CH3 CH CH3 iso-Propyl iso-Pr CH3CH2CH2CH2 n-Butyl n-Bu CH3CH2 CH CH3 sec-Butyl sec-Bu CH3CHCH2 CH3 iso-Butyl iso-Bu tert-Butyl tert-Bu n-Butane Isobutane CH3CH2CH2CH3 CH3CHCH3 CH3 CH3 C CH3 CH3 NOTE: n : normal sec: secondary tert: tertiary iso, sec, and tert are sometimes abbreviated as i-, s-, and t-. nomenclature(S09).docx -1- February 5, 2009 Laura B. Sonnichsen 5. Chemistry 203 IUPAC Names: (Hydrocarbons) In the IUPAC system, a name consists of (L)-#-substituents+prefix+suffix. Steps for naming a molecule: A. Find the largest carbon chain. This chain is the main chain, and the prefix. a. Example: a six carbon main chain has the prefix hex. b. EXCEPTION: If an unstaturation (double or triple bond) is present, the chain containing the unsaturation is ALWAYS the main chain (prefix). c. Rings: i. Use cyclo in front of the prefix to identify the presence of a ring. ii. If both a ring and a straight chain are present, whichever has the most number of carbons is the main chain (prefix). iii. EXCEPTION: If a phenyl (benzene) ring is present with a carbon chain of two or more that contains any other groups, the phenyl ring is the substituent. B. Determine the suffix. This is determined by examining the class of the compound. C. Number the main chain, giving the substituents the lowest possible numbers. a. Chains must be numbered starting at one end. Rings should always be numbered starting at an unsaturation or substituent. b. If an unsaturation is present, it should have the lowest possible number. c. If there are only alkyl substituents, then use the following method to determine the numbering: i. Number the chain in both directions. ii. Locate the first substituent going in both directions. iii. Which number is lower? This is the direction the chain should be numbered. iv. If both are the same, continue to the second substituent and repeat. If needed, keep going until the first numbering difference is reached. d. If there are two ways to number the chain, then use alphabetical order to determine the numbering. D. Write name! a. Write as: (L)-#-substituentprefixsuffix. b. If more than one substituent of a particular type, use di, tri, tetra, etc. to indicate the how many of that group. List all the position numbers in front of the substituent. c. If more than one type of substituent, they are listed alphabetically (NOT in number order!) d. If more than one unsaturation of a particular type, use di, tri, tera, etc. to indicate how many of that unsaturation. Di, tri, tetra, etc. are given between the prefix and the suffix (leave an extra “a” before the di, etc. as a spacer). List all unsaturation position numbers before the prefix and after any substituents. Example: 1,3-butadiene. e. USING ALPHABETICAL ORDERING: i. When ordering groups alphabetically, do NOT count di, tri, tetra, or other indicators showing the number of substituents. Also, tert- and sec- are ignored. ONLY iso and neo are counted. f. NOTE: Separate numbers with commas; numbers and letters with hyphens. g. The (L) part of the name is used to indicate configurational stereoisomers. i. For alkenes, use E or Z, where appropriate. If a molecule is E or Z, you must always include it in the name. ii. In common names, you might see cis or trans (italicized) instead of E or Z. Cis & trans don’t use the parantheses. nomenclature(S09).docx -2- February 5, 2009