Chemistry 221 - Test 3 Review Sheet
advertisement
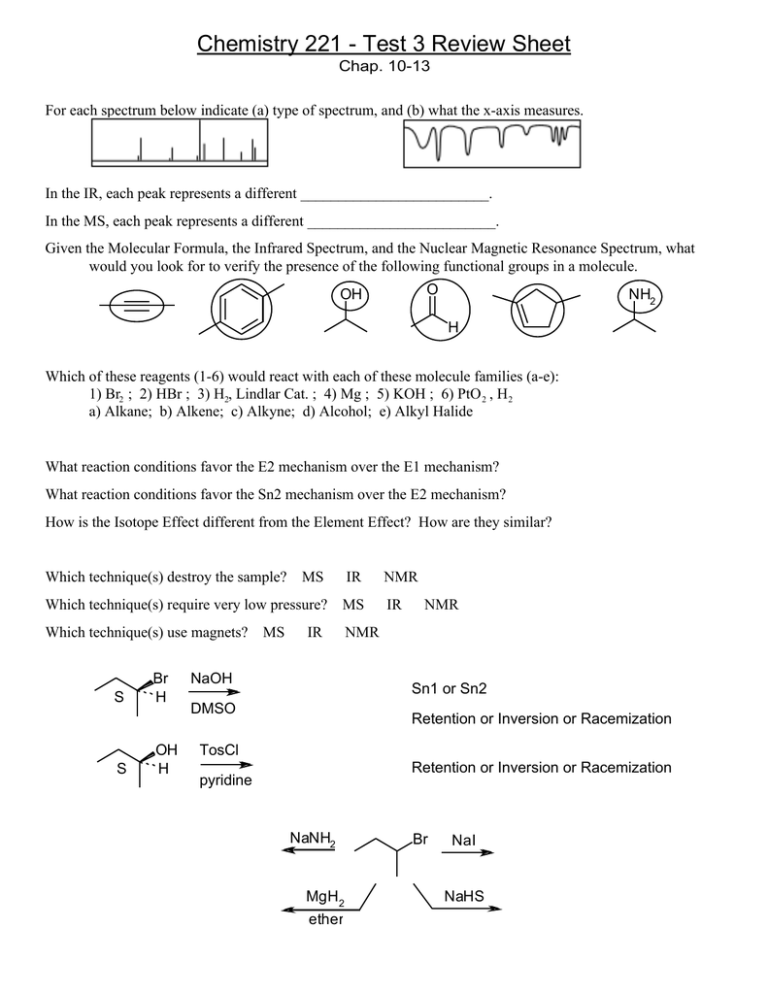
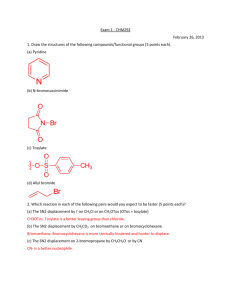
Chemistry 221 - Test 3 Review Sheet Chap. 10-13 For each spectrum below indicate (a) type of spectrum, and (b) what the x-axis measures. In the IR, each peak represents a different _________________________. In the MS, each peak represents a different _________________________. Given the Molecular Formula, the Infrared Spectrum, and the Nuclear Magnetic Resonance Spectrum, what would you look for to verify the presence of the following functional groups in a molecule. O OH NH2 H Which of these reagents (1-6) would react with each of these molecule families (a-e): 1) Br2 ; 2) HBr ; 3) H2, Lindlar Cat. ; 4) Mg ; 5) KOH ; 6) PtO 2 , H2 a) Alkane; b) Alkene; c) Alkyne; d) Alcohol; e) Alkyl Halide What reaction conditions favor the E2 mechanism over the E1 mechanism? What reaction conditions favor the Sn2 mechanism over the E2 mechanism? How is the Isotope Effect different from the Element Effect? How are they similar? Which technique(s) destroy the sample? MS IR NMR Which technique(s) require very low pressure? MS IR Which technique(s) use magnets? NMR S S Br H OH H MS IR NaOH NMR Sn1 or Sn2 DMSO Retention or Inversion or Racemization TosCl Retention or Inversion or Racemization pyridine NaNH2 MgH2 ether Br NaI NaHS