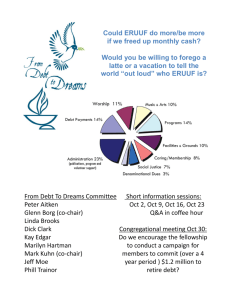
November 18, 2013
advertisement

November 18, 2013 Electron Configurations of Ions of the Representative Elements Sample Exercise 8.2 Predict the ion generally formed by (a) Sr (b) S (c) Al Oct 23­5:30 PM Oct 23­5:37 PM Transition Metal Ions Practice Exercise 8.2 Page 307 Predict the charges on the ions formed when magnesium reacts with nitrogen • Configuration for iron (II) vs iron (III) • Which element forms a 1+ ion that has the electron configuration [Kr] 4d8 ? Oct 23­5:38 PM Oct 23­5:39 PM Covalent Bonding Polyatomic Ions • Simplest example is H 2 molecule Oct 23­5:53 PM Oct 23­5:57 PM November 18, 2013 Lewis Structures • Hydrogen molecule • Chlorine molecule Sample Exercise 8.3 Given the Lewis symbols for nitrogen and fluorine shown in Table 8.1, predict the formula of the stable binary compound (a compound composed of two elements) formed when nitrogen reacts with fluorine, and draw its Lewis structure. EXAMPLES: • Hydrogen fluoride, Water, ammonia, methane Oct 23­6:00 PM Practice Exercise 8.3 (page 311) Compare the Lewis symbol for neon with the Lewis structure for methane (CH 4). In what important way are the electron arrangements about the neon and the carbon alike? In what important way are they different? Oct 23­6:10 PM Multiple Bonds: EXAMPLES: carbon dioxide nitrogen molecule Oct 23­6:11 PM Oct 23­6:13 PM Bond Polarity and Electronegativity: Bond Length of Covalent Compounds: Oct 23­6:17 PM Oct 23­6:22 PM November 18, 2013 Polarity of Bonds: Electronegativity Oct 23­6:26 PM Oct 23­6:29 PM Oct 23­6:34 PM Oct 23­11:25 PM Electronegativity Scale: Electronegativity and Bond Polarity Electronegativity Difference Type of Bond < 0.5 nonpolar 0.5 ­ 2.0 polar > 2.0 ionic • Consider: F 2 vs. HF vs. LiF Oct 23­6:39 PM Oct 23­6:43 PM November 18, 2013 Sample Exercise 8.4 In each case, which bond is more polar: (a) B­Cl or C­Cl (b) P­F or P­ Cl? Indicate in each chase which atom has the partial negative charge. Practice Exercise 8.4 (page 314) Which of the following bonds is most polar: S­Cl, S­Br, Se­Cl, Se­Br? Oct 23­6:46 PM Oct 23­6:48 PM Dipole Moments: • EXAMPLE: HF (polarity is indicated in two ways) • μ = Qr Q is the magnitude of the charge and r is the separation of the charges Oct 23­6:49 PM Oct 23­6:59 PM Oct 23­7:03 PM Oct 23­7:08 PM November 18, 2013 Bond Types and Nomenclature A Little More on Nomenclature: • Example TiO2 • Reason? • Question: The compounds MnO 3 and OsO 4 are more properly named molybdenum (VI) oxide and osmium tetroxide, respectively. Which of these compounds do you think has the higher melting point? Oct 23­7:10 PM Steps for Drawing Lewis Structures: Oct 23­7:23 PM Practice Exercise 8.6 (page 319) a) How many valence electrons should appear in the Lewis structure for CH 2Cl2? Oct 23­7:19 PM Sample Exercise 8.6 Draw the Lewis structure for phosphorus trichloride. Oct 23­7:29 PM Sample Exercise 8.7 Draw the Lewis structure for HCN. b) Draw the Lewis structure Oct 23­7:30 PM Oct 23­7:32 PM November 18, 2013 Practice Exercise 8.7 (page 319) Draw the Lewis structure for (a) NO + ion and (b) C 2H4 Oct 23­7:33 PM Sample Exercise 8.8 Draw the Lewis structure for the BrO 3­ ion. Oct 23­7:34 PM Formal Charge Practice Exercise 8.8 (page 320) Draw the Lewis structure for (a) ClO 2­ ion and (b) PO 43­ ion. • To calculate formal charge: Oct 23­7:35 PM Oct 23­7:36 PM Calculate the formal charge on the atoms in the CN ­ ion: To choose the most correct structure: 1) Choose the structure in which the atoms bear formal charges closet to zero Sulfate ion: 2)Choose the structure in which any negative charges reside on the more electronegative atoms 3) Choose the structure that obeys the octet rule if possible Note that the sum of the formal charges equals the overall charge of the ion. IMPORTANT REMINDER: Formal charges do not represent real charges on atoms Calculate the formal charge of the CO 2 structures that obey the octet rule (1 with 2 double bonds and 1 with 1 triple bond) Oct 23­7:44 PM Oct 23­7:46 PM November 18, 2013 Sample Exercise 8.9 The following are three possible structures for the thiocyanate ion (SCN­) Practice Exercise 8.9 (page 321) The cyanate ion (CNO ­), like the thiocyanate ion, has three possible Lewis structures. (a) Draw these three Lewis structures, and assign formal charges to the atoms in each structure. (b) Which Lewis structure is the preferred one? (a) Determine the formal charges of the atoms in each structure (b) Which Lewis structure is the preferred one? Oct 23­7:54 PM Oct 23­7:56 PM Green Paint Analogy Resonance Structures • Consider the structure of the ozone molecule • Resonance structures are Oct 23­7:58 PM Resonance structures of the nitrate ion: Oct 23­8:04 PM Oct 23­8:02 PM Sample Exercise 8.10 Which is predicted to have shorter sulfur­oxygen bonds, SO 3 or SO32­ ? Oct 23­8:05 PM November 18, 2013 Practice Exercise 8.10 (page 324) Draw two equivalent resonance structures for the formate ion, HCO 2 ­ . Resonance in Benzene Benzene is an aromatic organic compound with the formula C 6H6. The six C atoms are bonded in a hexagonal ring and a H is bonded to each C. Two resonance structures for benzene that satisfy the octet rule: Shorthand version: The bonding in benzene gives it a special stability. This results in millions of organic compounds having the characteristic 6­C ring structure. These compounds are important in pharmaceuticals, biochemistry, and production of modern materials. Oct 23­8:07 PM Oct 23­8:08 PM Odd Number of Electrons Occurs in: Exceptions to the Octet Rule: The octet rule fails in many situations involving covalent bonds: ClO2 NO NO2 O2­ Oct 23­8:13 PM Oct 23­8:16 PM Less than 8 Valence Electrons: • Most often found in compounds of Boron and Beryllium • Consider boron trifluoride (B has only 6 electrons) More than 8 Valence Electrons: • PCl 5 requires 10 electrons around the central phosphorus atom • This bonds easily with molecules having an unshared pair of electrons (like ammonia) • Will form NH 3BF3 Oct 23­8:18 PM Oct 23­8:22 PM November 18, 2013 Sample Exercise 8.11 Draw the Lewis structure for ICl 4­. Oct 23­8:26 PM Strength of Covalent Bonds: Oct 23­8:36 PM Practice Exercise 8.11 (page 328) (a) Which of the following atoms is never found with more than an octet of electrons around it: S, C, P, Br? (b) Draw the Lewis structure for XeF2. Oct 23­8:34 PM Bond Enthalpy and Enthalpy of Reaction: Oct 23­8:45 PM Determining Enthalpies of Reactions: Consider two steps: Oct 23­8:49 PM Oct 23­11:34 PM November 18, 2013 Consider the reaction: H­CH3(g) + Cl­Cl(g) ­­> Cl­CH 3(g) + H­Cl(g) ΔHrxn =? Sample Exercise 8.12 Using Table 8.3, estimate ΔH for the following reaction (where we explicitly show the bonds involved in the reactants and products): Important factors to remember: Bond enthalpies are given for gaseous molecules and are averaged values Oct 23­8:52 PM Practice Exercise 8.4 (page 332) Using Table 8.4, estimate ΔH for the reaction: Oct 23­10:30 PM Oct 23­9:00 PM Bond Enthalpy and Bond Length In general, as the number of bonds between two atoms increases, the bond grows shorter and stronger. Oct 23­11:01 PM Sample Integrative Exercise Page 334 Phosgene, a substance used in poisonous gas warfare in World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek word phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14%C, 16.17%O, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol. (a) Determine the molecular formula of this compound. (b) Draw three Lewis structures for the molecule that satisfy the octet rule for each atom..the Cl and O atoms bond to the C. (c) Using formal charges determine which Lewis structure is the most important one. (d) Using average bond enthalpies, estimate ΔH for the formation of gaseous phosphene from CO(g) and Cl2(g). Oct 23­11:04 PM Oct 23­11:11 PM November 18, 2013 Oct 23­6:41 PM