Steam Distillation: Carvone & Limonene Lab - Organic Chemistry
advertisement

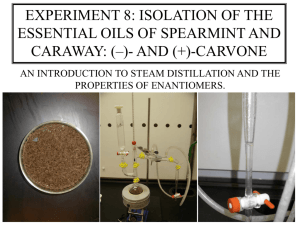
CHEM 233: Organic Laboratory I Prelab Lecture University of Illinois at Chicago UIC O H (R)-Carvone Lab 5: Steam Distillation of Monoterpenes Carvone and Limonene from Caraway Seeds. Analysis of Products by Infrared Spectroscopy and TLC. O H (S)-Carvone Midterm Exam Reminder Midterm Exam = _ • Sample exam online: www.chadlandrie.com (shared files page). • Review past homework questions & assigned reading. • Review background info provided in course manual. • Review prelab lecture notes. • Exam will be given during your lab time in the same room. • Topics cover both theory and techniques of lab experiments. University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 2 Prelab Lecture: Lab 5 Steam Distillation: Applications & Goals Applications: Today’s Goals: 1. Last separation/ purification technique in part one of CHEM 233 course. 1. Isolate carvone from caraway seeds by steam dist. Determine enantiomer. 2. Industrial = isolation of volatile essential oils from plant material. 2. Obtain IR of isolated oil; compare to known IRs of carvone. 3. General = isolation of oils/liquids with high bps. 3. Obtain TLC of isolated oil with a co-spot of authentic. University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 3 Prelab Lecture: Lab 5 Steam Distillation: Advantages & Requirements Advantages: Requirements: 1. Distill liquids with high boiling points at temperatures < 100ºC. 1. Non-reactive with H2O. 2. Avoid high temp simple/ fractional distillation, which could cause decomposition. 3. Stable (does not decompose) at 100 ºC. 2. Immiscible with H2O. 4. Pº ≥ 5 Torr at 100 ºC. 3. Alternative = vacuum dist. University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 4 Prelab Lecture: Lab 5 Steam Distillation Apparatus separatory funnel (used here as a dropping funnel) Notes: 1. No need for boiling stones; caraway seeds provide sufficient sites for nucleation. 2. Steam is generated in situ by boiling water in the stillpot. 3. Replacement water (hot) is added at the same rate distillate is exiting the condenser. replacement water (hot!!) stillhead Claisen adapter water & caraway seeds University of Illinois at Chicago UIC 4. Separatory funnel is actually being used during the distillation as a dropping funnel. 5. Collect approximately 75 mL of distillate. Distillate will be cloudy since carvone and water are immiscible. 6. Volatile components in the caraway seeds are co-distilling with the water vapor. © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 5 Prelab Lecture: Lab 5 Initial Considerations O O H H (R)-Carvone O H H (S)-Carvone water 1. Which compound above has the highest Pº? Lowest? 2. In a mixture of water and carvone, which would you expect to distill out first? Why? 3. Do you expect carvone to be soluble in water? Why or why not? University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 6 Prelab Lecture: Lab 5 Principles: Mathematical immiscible = not soluble in all proportions For immiscible component (x) in a heterogeneous mixture with H2O: Raoult’s Law: Px = Nx * Pxº Dalton’s law still applies: PT = Pxº + PH2Oº or PT = Px + PH2O bp (mixture): PT = Patm Px = Pxº Since x is not soluble in water, it does not depend on its mole fraction in the mixture. This relationship applies to each component in mixture, including water. University of Illinois at Chicago UIC Conclusion: The total vapor pressure (PT) is always higher than the most volatile component-always H2O in steam distillation. The bp of the mixture is always lower than the lowest boiling component--again, always H2O (100ºC) in steam distillation. © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 7 Prelab Lecture: Lab 5 Principles: Graphical Vapor Pressure-Temperature Diagram for Water, Carvone and Limonene 900 atmospheric pressure 800 760 Pressure (Torr) 700 O PT PH2O 600 500 400 300 200 Plim Plim 100 0 0 50 100 150 Temperature 200 250 (0C) boiling point (< 100 ºC): PT = (PH2O + Plim + Pcar) = 760 Torr University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 8 Prelab Lecture: Lab 5 Procedure Notes on Extraction • Your TA will demonstrate proper use of a separatory funnel for extractions. H2O layer (d = 1.0 g/mL) CH2Cl2 layer (d = 1.33 g/mL) • CH2Cl2 and water are immiscible. • Less dense liquid (H2O in this case) is top layer. • Like dissolves like; therefore, carvone (organic) is soluble in CH2Cl2 (organic). University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 9 Prelab Lecture: Lab 5 Analysis of Isolated Oil Thin-Layer Chromatography Infrared Spectroscopy • Compare Rf value of isolated oil with authentic sample of carvone. • Find a solvent system (mixture of EtOAc & hexanes) where Rf < 0.6. • Use a co-spot. • Are the Rf values of the isolated and authentic samples of carvone the same or different? What does the result imply? • Is the oil mainly one component or a mixture of many components? • Obtain IR of isolated oil. You may need volatiles cover since carvone is, well, volatile. isolated oil (dilute in small amount of CH2Cl2) University of Illinois at Chicago co-spot UIC authentic sample of carvone • Compare your IR spectra with those of carvone on next slides. • Identify singnals for C=C, C=O in your spectra. Smell!! Does your oil smell like spearmint [(R)-carvone] or does it smell like caraway [(S)-carvone]? Consult with your friends; maybe the receptors in your nose can’t tell the difference!! IR and TLC cannot tell you which enantiomer of carvone you’ve isolated--only your nose. Optical rotation can differentiate between enantiomers, but we do not have a polarimeter. © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 10 Prelab Lecture: Lab 5 IR of Both Enantiomers of Carvone (S)-(+)-Carvone (S)-carvone is the enantiomer of (R)-carvone Enantiomers have the same physical properities including IR vibrational frequencies, mp, bp, Rf, etc. (R)-(-)-Carvone IR spectra for both enantiomers of carvone-and for any two enantiomeric compounds--are identical. University of Illinois at Chicago UIC © 2009, Dr. Chad L. Landrie CHEM 233: Organic Chemistry Laboratory 1 Slide 11 Prelab Lecture: Lab 5