Research
www. AJOG.org
ONCOLOGY
Comparison of molecular assays for detection
and typing of human papillomavirus
Christoph Koidl, MD; Michael Bozic; Ita Hadzisejdic, MD; Maja Grahovac, MD; Blazenka Grahovac, PhD;
Wolfgang Kranewitter, PhD; Egon Marth, MD, PhD; Harald H. Kessler, MD
OBJECTIVE: The objective of the study was to compare the performance of 3 different extraction instruments in conjunction with 4 different amplification and detection kits for detection and typing of human papillomavirus (HPV) deoxyribonucleic acid (DNA).
typing test. After extraction on the easyMAG instrument, 32 samples
tested positive when the PapilloCheck with the HotStarTaq DNA polymerase was used.
RESULTS: In 31 samples, HPV DNA was detected by both the Amplicor
CONCLUSION: Together with extraction on the easyMAG instrument,
the Amplicor HPV test, the LINEAR ARRAY HPV genotyping test, and
the new PapilloCheck with the HotStarTaq DNA polymerase provide
comparable results allowing reliable and safe HPV diagnostics in the
routine laboratory. Use of alternative assays may lead to an increase of
invalid and divergent HPV typing results.
HPV test and the LINEAR ARRAY HPV genotyping test in conjunction
with DNA extraction on the easyMAG instrument. In another 6 samples,
only low-risk types were detected with the LINEAR ARRAY HPV geno-
Key words: DNA extraction, human papillomavirus, microarray,
polymerase chain reaction, typing
STUDY DESIGN: A total of 42 cervical swabs were investigated. HPV
DNA was extracted on the 3 different instruments. Each of the extracts
was then amplified, and HPV DNA amplification products were detected with 4 different kits.
Cite this article as: Koidl C, Bozic M, Hadzisejdic I, et al. Comparison of molecular assays for detection and typing of human papillomavirus. Am J Obstet Gynecol
2008;199:144.e1-144.e6.
T
he human papillomavirus (HPV)
has been found to be responsible for
development of cervical cancer and its
associated precancerous lesions.1,2 Cervical cancer is the most common female
cancer in developing countries with an
estimated global incidence of approximately 470,000 cases and 233,000 deaths
per year.3,4 Because of organized cervical
screening programs in developed countries that detect early stages of this disease, the incidence of fatal cases is significantly lower.5
Fixed-interval surveillance of women
with cervical cytology screening based either on Papanicolaou staining of epithelial cells or liquid-based cytology sampled from the cervix is currently 1 of the
most successful cancer screening programs. However, cervical cytology lacks
sensitivity; moreover, no international
consensus with regard to the overall sensitivity of classical cytology for the detection of histologically suspicious areas of
the cervix has been established.6-8 Therefore, cervical cancer screening strategies
From the Molecular Diagnostics Laboratory, Institute of Hygiene, Medical University of
Graz, Graz, Austria (Drs Koidl, Marth, and Kessler and Mr Bozic); the Department of
Pathology, Medical Faculty University of Rijeka, Rijeka, Croatia (Drs Hadzisejdic and B.
Grahovac); the Department of Dermatology and Venerology, Clinical Hospital Center
Zagreb, Zagreb, Croatia (Dr M. Grahovac); and the Institute of Laboratory Medicine, General
Hospital Linz, Linz, Austria (Dr Kranewitter).
Received Aug. 20, 2007; revised Dec. 17, 2007; accepted March 3, 2008.
Reprints: Professor Dr Harald H. Kessler, Molecular Diagnostics Laboratory, Institute of Hygiene,
Medical University of Graz, Universitaetsplatz 4, A-8010 Graz, Austria. harald.kessler@medunigraz.at.
This study was supported in part by grants from the Austrian-Croatian Scientific and Education
Cooperation Action Program and from Lambda GmbH.
0002-9378/$34.00 • © 2008 Mosby, Inc. All rights reserved. • doi: 10.1016/j.ajog.2008.03.005
144.e1
American Journal of Obstetrics & Gynecology AUGUST 2008
based on molecular HPV testing have
been introduced. Depending on time,
cost, organization, and different national
screening policies, molecular HPV testing has been implemented in fixed-interval surveillance of women in recent
years.9
More than 90 different HPV genotypes have been cloned and officially designated. More than 40 HPV genotypes
may infect the human anogenital tract
and 15 of these HPV types are classified
as high-risk (HR) types for the development of cervical cancer and its precancerous lesions.10,11 For direct detection
and typing of HPV in clinical samples,
molecular assays have been introduced
recently. Currently 3 commercially
available assays, the Hybrid Capture 2
(Digene Corp, Gaithersburg, MD), the
Amplicor HPV test (Roche Molecular
Systems, Inc, Branchburg, NJ), and the
LINEAR ARRAY HPV genotyping test
(Roche) assays have been evaluated extensively and found to be appropriate for
use in primary screening and triage.12-15
In the present study, 42 cervical swabs
were investigated. The performance of 3
Oncology
www.AJOG.org
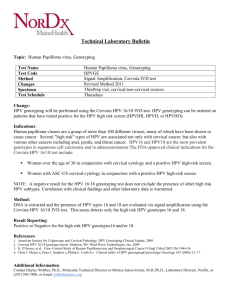
FIGURE 1
Study flow diagram
HPV DNA was extracted out of each sample with 3 different instruments. For amplification and
detection of HPV DNA, 4 different kits were used.
Koidl. Comparison of molecular assays in human papillomavirus. Am J Obstet Gynecol 2008.
different methods for extraction of HPV
DNA in conjunction with 4 different
methods for PCR amplification and detection of amplification products were
compared.
HPV amplification and detection assays,
yielding a total of 504 results (Figure 1).
Results obtained by different assay were
compared.
Sample preparation
M ATERIALS AND M ETHODS
Samples
A total of 42 cervical swabs obtained
from women with persistent Papanicolaou III D status in cervical cytology were
studied. Cervical swabs were taken with
Cervex-Brush cervical cell sampler (Rovers Medical Devices B.V., Oss, The Netherlands) and collected in 20 mL ThinPrep PreservCyt solution transport
medium (Cytec Corp, Boxborough,
MA) according to the manufacturer’s instructions. Samples were stored at 4°C
until analysis within 3 weeks after
collection.
Study design
HPV deoxyribonucleic acid (DNA) was
extracted from 42 cervical swabs with 3
different commercially available methods yielding a total of 126 extracts. Extracts were investigated with 4 different
Automated HPV DNA extraction was
performed on each of 3 commercially
available sample preparation instruments: the easyMAG (bioMerieux sa,
Marcy l’Etoile, France), the MagNA Pure
LC (Roche Applied Science, Mannheim,
Germany), and the BioRobot EZ1 workstation (QIAGEN, Hamburg GmbH,
Hamburg, Germany).
When HPV DNA was extracted on the
easyMAG, the NucliSens easyMAG accessory products (bioMerieux) were
used. A total of 400 L of the transport
medium were pipetted into the
easyMAG reaction tube. Samples were
prepared with the automated extraction
protocol program Generic 1.0.6 (bioMerieux) including an additional manual external lysis step (4 times stirring of
the sample with a pipette). The elution
volume was set to be 110 L.
Research
Prior to automated HPV DNA extraction on the MagNA Pure LC, 400 L of
the sample transport medium were centrifuged with 12,000 rpm for 5 minutes at
room temperature. After discarding the
supernatant, the pellet was resuspended
in 190 L of tissue lysis buffer (Roche
Applied Science) followed by addition of
10 L of Proteinase K (Roche Applied
Science). After incubation on a mixing
platform for 3 hours at 56°C, the sample
was transferred into the MagNA Pure LC
reaction tube. On the MagNA Pure LC,
HPV DNA was extracted with the
MagNA Pure DNA Isolation Kit I
(Roche Applied Science). The DNA I
high-performance external lysis program (Roche Applied Science) was used.
The elution volume was set to be 200 L.
Prior to automated HPV DNA extraction on the BioRobot EZ1 workstation,
200 L of the sample transport medium
were centrifuged with 12000 rpm for 5
minutes at room temperature. After discarding the supernatant, the pellet was
resuspended in 190 l buffer G2 (QIAGEN) followed by the addition of 10 L
Proteinase K (QIAGEN). After incubation on a mixing platform for 3 hours at
56°C, the sample was transferred into the
BioRobot EZ1 reaction tube. On the
BioRobot EZ1 workstation, HPV DNA
was extracted with the EZ1 DNA tissue
kit (QIAGEN) using the EZ1 DNA tissue
card (QIAGEN). The elution volume
was set to be 100 L.
Amplification and detection
of HPV DNA
Amplification and detection of HPV
DNA were performed with each of 4 assays: the Amplicor HPV test (Roche Applied Science), the LINEAR ARRAY
HPV genotyping test (Roche Applied
Science), the PapilloCheck (Greiner BioOne GmbH, Frickenhausen, Germany)
with AmpliTaq Gold (Applied Biosystems, Foster City, CA), and the PapilloCheck (Greiner Bio-One) with HotStarTaq DNA polymerase (QIAGEN).
The Amplicor HPV test is designed for
qualitative detection of HPV DNA. It
provides detection of HR HPV types 16,
18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59,
and 68 without typing. When HPV DNA
was amplified with the Amplicor HPV
AUGUST 2008 American Journal of Obstetrics & Gynecology
144.e2
Research
Oncology
test, the Gene Amp PCR System 9700
(Applied Biosystems) was used according to the manufacturer’s instructions.
Fully automated hybridization and detection of HPV DNA was done on the
BEP III (Dade Behring Marburg GmbH,
Marburg, Germany).
The LINEAR ARRAY HPV genotyping test is designed for qualitative detection and typing of HPV types 6, 11, 16,
18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 53, 54,
55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70,
71, 72, 73, 81, 82, 83, 84, CP6108, and
IS39. When HPV DNA was amplified
with the LINEAR ARRAY HPV genotyping test, the Gene Amp PCR System 9700
was used according to the manufacturer’s instructions. Fully automated hybridization and detection of HPV DNA
was done on the ProfiBlot 48 (Tecan
Trading AG, Zurich, Switzerland).
The PapilloCheck is designed for qualitative detection and typing of HPV types
6, 11, 16, 18, 31, 33, 35, 39, 40, 42, 43, 44,
45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73,
and 82. The PapilloCheck master mix
does not include the DNA polymerase.
In this study, either the AmpliTaq
Gold or the HotStarTaq DNA polymerase was added to the master mix. When
the HPV DNA was amplified with the
PapilloCheck, the Gene Amp PCR System 9700 thermocycler was used according to the manufacturer’s instructions.
After hybridization, analysis of the PapilloCheck DNA chip was performed on
the CheckScanner (Greiner Bio-One)
using the CheckReport (Greiner BioOne) analysis software according to the
package inserts.
Both the Amplicor HPV test and the
LINEAR ARRAY HPV genotyping test
include a primer pair for amplification
and detection of the human beta-globin
gene as heterologous internal control for
cell adequacy, extraction, and amplification. The PapilloCheck includes both a
primer pair for amplification and detection of the human ADAT1 gene as heterologous control for cell adequacy, extraction, and amplification and another
primer pair for the amplification and detection of an artificial control template as
an additional control for amplification.
If any of the internal controls were not
144.e3
www.AJOG.org
detected, the result was considered as
invalid.
R ESULTS
Of 42 cervical swabs, HPV DNA was not
detected in 5 samples with any of the
tests used. In 31 samples, HPV DNA was
detected by the Amplicor HPV test and
the LINEAR ARRAY HPV genotyping
test, both in conjunction with DNA extraction on the easyMAG instrument. All
of these samples contained either only
HR HPV types or a combination of HR
and low-risk (LR) HPV types. In another
6 samples, only LR HPV types were
found with the LINEAR ARRAY HPV
genotyping test. With both of these assays, the heterologous internal control
was consistently detected.
When the Amplicor HPV test was used
in conjunction with DNA extraction on
the MagNA Pure LC instrument, 26 of 37
samples with HR HPV types tested positive. One of the positives contained only
LR HPV types and was thus considered
to be false positive. Three samples tested
negative and 8 gave an invalid result.
When the Amplicor HPV test was used
in conjunction with DNA extraction on
the BioRobot EZ1 workstation, 14 of 37
samples with HR HPV types tested positive. One of the positive samples contained only LR HPV types and was thus
considered to be false positive. Four
samples tested negative. Two of the samples contained an HR HPV type and
were thus considered to be false negative.
The remaining 19 samples gave an invalid result.
When the LINEAR ARRAY HPV
genotyping test was performed for HPV
DNA amplification and typing after
DNA extraction with the easyMAG, a
single or more HPV types were found in
37 samples. The heterologous internal
control was consistently detected in all
samples. After DNA extraction with the
MagNA Pure LC instrument, 29 samples
with a single or more HPV types, 2 false
negatives and 6 invalid results were obtained. After DNA extraction with the
BioRobot EZ1 workstation, 11 samples
with a single or more HPV types, 9 false
negatives, and 17 invalid results were
obtained.
American Journal of Obstetrics & Gynecology AUGUST 2008
FIGURE 2
Number of invalid results when
using different extraction
instruments
Number of invalid results when using different
extraction instruments in conjunction with kits
for qualitative detection and typing of HPV
types. x-axis, kits used in this study; y-axis,
extraction instruments used in this study.
Koidl. Comparison of molecular assays in human
papillomavirus. Am J Obstet Gynecol 2008.
With the LINEAR ARRAY HPV genotyping test, 2 samples were found to contain only bands CP6108 and HPV 62, respectively. Because these HPV types are
not detectable with the PapilloCheck assay, valid negative results for those samples obtained by the PapilloCheck assay
were referred to as true negative. When
the PapilloCheck with the AmpliTaq
Gold DNA polymerase in the master mix
was performed for HPV DNA amplification and typing after DNA extraction
with the easyMAG, a single or more HPV
types were obtained in 30 samples. Of the
remaining 7 samples, 2 were true negative (see aforementioned text), 3 were
false negative, and 2 gave invalid results.
After DNA extraction with the MagNA
Pure LC instrument, a single or more
HPV types were obtained in 12 samples.
Of the remaining 25 samples, 1 was false
negative and 24 gave invalid results. After DNA extraction with the BioRobot
EZ1 workstation, a single or more HPV
types were obtained in 20 samples. Of the
remaining 17 samples, 1 was true negative (see aforementioned text), 3 were
false negative, and 13 gave invalid results.
When the PapilloCheck with the HotStarTaq DNA polymerase in the master
mix was performed for HPV DNA amplification and typing after DNA extraction with the easyMAG, a single or more
Results obtained with HPV assays for qualitative detection and typing of HPV types
easyMAG
Sample
no.
MagNA Pure LC
BioRobot EZ1
Linear Array
PapilloCheck/
AmpliTaq
PapilloCheck/
HotStarTaq
Linear Array
PapilloCheck/
AmpliTaq
PapilloCheck/
HotStarTaq
Linear Array
PapilloCheck/
AmpliTaq
PapilloCheck/
HotStarTaq
1
18
18, 45
18, 45
18
18, 45
18, 45
18
18
18, 45, 70
2
16, 33, 42, 53, 59, 66
16, 42, 43, 59
16, 33, 42, 53, 56, 59
16, 42, 53, 59, 66
42, 53, 59
16, 42, 53, 56,
59
Invalid
42, 53
16, 42, 53, 56,
59, 66, 70, 73
www.AJOG.org
TABLE
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
3
66
False negative
False negative
False negative
False negative
False negative
Invalid
False negative
False negative
4
66, 56
56, 66
54, 55 56, 66
66
Invalid
66
66
66
44, 45, 56, 59,
66, 70, 73, 82
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
5
16
Invalid
Invalid
Invalid
Invalid
Invalid
16
Invalid
False negative
6
31
31
31
31
Invalid
45
31
31
31
7
16
16
16
16
16, 45
16, 56
16
16
16, 56
8
59
59
59
59
Invalid
Invalid
False negative
59
59, 73
9
31
31
31
31
Invalid
31
Invalid
Invalid
31
10
18, 52
18, 52
18, 52
18, 52
45, 52
18, 52
18, 52
18, 52
18, 52
11
33, 52
33
33
33
Invalid
Invalid
33
33
33
12
16
16
16
16
Invalid
45
16
False negative
16
13
70
False negative
False negative
70
Invalid
Invalid
70
Invalid
False negative
14
CP6108
True negative
True negative
False negative
Invalid
Invalid
CP6108
Invalid
True negative
15
16, 31, 52, 56
16, 56
16, 31, 56, 66
56
Invalid
Invalid
False negative
Invalid
16, 56
16
6, 66, 84
66
56, 59, 66
66, 84
66
56, 66
False negative
Invalid
66
17
16
16
16
Invalid
Invalid
Invalid
False negative
Invalid
Invalid
18
16, 31
16, 31
16, 31
31
Invalid
31
False negative
31
16, 31, 66
19
53, 54, 61, 66, 82, 84
56, 66, 82
53, 56, 66, 82
53, 54, 61, 66, 82, 84
45, 66, 82
53, 56, 66, 82
66
Invalid
66, 82
20
52, CP6108
Invalid
52
Invalid
Invalid
Invalid
False negative
Invalid
52
21
39
39
39
39
Invalid
Invalid
False negative
Invalid
39
22
51
51
51
51
51
51
False negative
51
51
23
31, 62, 66
66
31, 56, 66
31, 62, 66
66
31, 56, 66
Invalid
Invalid
56, 66
24
66
66
56, 66
Invalid
Invalid
66
Invalid
66
56, 66
25
16, 31, 42, 59, 66
42, 59
16, 31, 42, 59, 66
59
Invalid
42, 59
Invalid
42, 59
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
a
a
a
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
31, 42, 59
144.e4
Continued on page 144.e5.
Research
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
Oncology
AUGUST 2008 American Journal of Obstetrics & Gynecology
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
144.e5
16, 81
16
33, 52, 81
31, 62, 81
58, 56
16, 31, 52, 59
16
62
16, 51, 66
52, 62
51, 53, 56, 58
56, 61, 66, 68, 73
26
27
28
29
30
31
32
33
34
35
36
37
16
PapilloCheck/
AmpliTaq
16
PapilloCheck/
HotStarTaq
PapilloCheck/
AmpliTaq
Invalid
Invalid
Invalid
Invalid
Invalid
Linear Array
Invalid
Invalid
33
31, 81
56
MagNA Pure LC
45
PapilloCheck/
HotStarTaq
Invalid
Linear Array
BioRobot EZ1
16
PapilloCheck/
AmpliTaq
16
PapilloCheck/
HotStarTaq
16
33, 51
Invalid
False negative
Invalid
Invalid
16
False negative
16
33
31
31
False negative
31
31
56
Invalid
Invalid
Invalid
56
Invalid
16
Invalid
52
56
56, 66
16
16
62
16, 51
52
53, 56, 58
56, 61, 66, 68, 73
True negative
16, 51, 66, 82
16, 31, 52
16, 82
Invalid
16, 45
Invalid
Invalid
Invalid
16
16, 31
16
Invalid
Invalid
Invalid
True negative
Invalid
16
Invalid
16, 51, 66, 82
Invalid
American Journal of Obstetrics & Gynecology AUGUST 2008
52
53, 56
52
53, 56
Invalid
Invalid
52
56
52
56
56, 66
Both HPV 6108 and HPV 62 not detectable by the PapilloCheck.
Koidl. Comparison of molecular assays in human papillomavirus. Am J Obstet Gynecol 2008.
a
45, 56, 66
Invalid
56, 66
56, 66
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
56
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
56
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
52, 31
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
16, 51
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
True negative
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
a
a
a
False negative
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
16, 31
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
56
......................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
31
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
33
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
16
............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
Linear Array
Sample
no.
easyMAG
Continued from page 144.e4.
Results obtained with HPV assays for qualitative detection and typing of HPV types
TABLE
Research
Oncology
www.AJOG.org
HPV types were obtained in 32 samples.
Of the remaining 5 samples, 2 were true
negative (see aforementioned text), 2
were false negative, and one gave an invalid result. After DNA extraction with
the MagNA Pure LC instrument, a single
or more HPV types were obtained in 22
samples. Of the remaining 15 samples, 2
were false negative and 13 gave invalid
results. After DNA extraction with the
BioRobot EZ1 workstation, a single or
more HPV types were obtained in 30
samples. Of the remaining 7 samples, 1
was true negative (see aforementioned
text), 3 were false negative, and 3 gave
invalid results.
Numbers of invalid results when using
different extraction instruments in conjunction with kits for qualitative detection and typing of HPV types are shown
in Figure 2. Detailed HPV typing results
are demonstrated in the Table.
When 8 samples were analyzed in parallel, DNA extraction including manual
pretreatment of samples required 50 min
with the easyMAG. When alternative extraction instruments were used, 70 minutes were required with the MagNA Pure
LC instrument and 230 minutes with the
BioRobot EZ1 workstation. For amplification and detection, the Amplicor HPV
test required 300 minutes, the LINEAR
ARRAY HPV genotyping test 340 minutes, and the PapilloCheck 210 minutes.
C OMMENT
Chronic infection with HPV is responsible for development of cervical cancer
and its associated precancerous lesions.1,2 In addition to classical cervical
screening with Papanicolaou staining or
liquid-based cytology, molecular detection of HPV DNA has been implemented
in fixed-interval surveillance of women
in recent years.9 For detection and typing
of HPV DNA, currently 3 commercially
available assays are predominantly used
for cervical surveillance and triage.12-15
In this study, the performance of 3 different methods for extraction of HPV in
conjunction with 4 different methods for
PCR amplification and detection of amplification products were compared. The
rationale of this study was to identify differences in the outcome of results.
Oncology
www.AJOG.org
When the Amplicor HPV test and the
LINEAR ARRAY HPV genotyping test
were used in conjunction with DNA extraction on the easyMAG instrument,
concordant results were obtained for all
samples. With the Amplicor assay in
conjunction with DNA extraction on the
MagNA Pure LC instrument, 1 of the LR
HPV DNA samples was found to be positive. This result was considered to be
false positive and may be explained by
carryover contamination because of the
open mode system of this extraction instrument. In contrast, both of the alternative extraction instruments used in
this study use closed mode systems to
minimize contamination risk during
sample preparation.
When the Amplicor HPV test and the
LINEAR ARRAY HPV genotyping test
were used in conjunction with DNA extraction on the easyMAG instrument, no
invalid results were found in all samples
tested. With alternative DNA extraction
instruments, both an increased number
of invalid results and false-negative samples were found, emphasizing the need of
inclusion of internal controls in molecular assays in general and the weakness of
alternative extraction instruments used
in this study.16
When the 3 HPV genotyping assays
used in this study were compared, HPV
typing results were found to be inhomogeneous in infections with multiple HPV
types. The performance of the PapilloCheck with the HotStarTaq DNA Polymerase in the master mix was found to be
superior to that with the AmpliTaq Gold
in the master mix.
For the laboratory workflow, sample
processing time is a major issue. Time
required for sample preparation includ-
ing manual steps and automated sample
processing on the easyMAG instrument
was found to be superior to that with
both the MagNA Pure LC instrument
and the BioRobot EZ1 workstation. For
HPV amplification and detection, the
PapilloCheck assay showed the best time
efficiency.
In conclusion, the Amplicor HPV test,
the LINEAR ARRAY HPV genotyping
test, and the new PapilloCheck with the
HotStarTaq DNA polymerase provide
comparable results when performed after extraction on the easyMAG instrument, allowing reliable and safe HPV diagnostics in the routine laboratory. Use
of alternative assays may lead to an increase of invalid results and divergent
f
HPV typing results.
ACKNOWLEDGMENT
The authors gratefully acknowledge Michael
Schleichert for stimulating discussions and Jörg
Berg for technical support.
REFERENCES
1. Bosch FX, Lorincz A, Munoz N, Meijer CJ,
Shah KV. The causal relation between human
papillomavirus and cervical cancer. J Clin
Pathol 2002;55:244-65.
2. Walboomers JM, Jacobs MV, Manos MM, et
al. Human papillomavirus is a necessary cause
of invasive cervical cancer worldwide. J Pathol
1999;189:1-3.
3. Einstein MH, Goldberg GL. Human papillomavirus and cervical neoplasia. Cancer Invest
2002;20:1080-5.
4. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan
2000. Int J Cancer 2001;94:153-6.
5. Parkin DM, Pisani P, Ferlay J. Estimates of
the worldwide incidence of 25 major cancers in
1990. Int J Cancer 1999;80:827-41.
6. Fahey MT, Irwig L, Macaskill P. Meta-analysis
of Pap test accuracy. Am J Epidemiol 1995;
141:680-9.
Research
7. Bernstein SJ, Sanchez-Ramos L, Ndubisi B.
Liquid-based cervical cytologic smear study
and conventional Papanicolaou smears: a
metaanalysis of prospective studies comparing
cytologic sample diagnosis and sample adequacy. Am J Obstet Gynecol 2001;185:
308-17.
8. Castle PE, Sadorra M, Garcia F, Holladay EB,
Kornegay J. A pilot study of a commercialized
human papillomavirus (HPV) genotyping assay:
comparison of HPV risk group to cytology and
histology. J Clin Microbiol 2006;44:3915-7.
9. Berkhof J, de Bruijne MC, Zielinski GD, et al.
Evaluation of cervical screening strategies with
adjunct high-risk human papillomavirus testing
for women with borderline or mild dyscakaryosis. Int J Cancer 2006;118:1759-68.
10. Munoz N, Bosch FX, de Sanjose S, et al.
Epidemiologic classification of human papillomavirus types associated with cervical cancer.
N Engl J Med 2003;348:518-27.
11. zur Hausen H. Papillomaviruses and cancer: From basic studies to clinical application.
Nat Rev Cancer 2002;2:342-50.
12. Giuliani L, Coletti A, Syrjanen K, Favalli C,
Ciotti M. Comparison of DNA sequencing and
Roche Linear Array in human papillomavirus
(HPV) genotyping. Anticancer Res 2006;
26:3939-41.
13. Monsonego J, Bohbot JM, Pollini G, et al.
Performance of the Roche AMPLICOR human
papillomavirus (HPV) test in prediction of cervical intraepithelial neoplasia (CIN) in women with
abnormal PAP smear. Gynecol Oncol 2005;
99:160-8.
14. Sandri MT, Lentati P, Benini E, et al. Comparison of the Digene HC2 assay and the Roche
AMPLICOR human papillomavirus (HPV) test
for detection of high-risk HPV genotypes in cervical samples. J Clin Microbiol 2006;44:2141-6.
15. Stevens MP, Rudland E, Garland SM,
Tabrizi SN. Assessment of MagNA pure LC extraction system for detection of human papillomavirus (HPV) DNA in PreservCyt samples by
the Roche AMPLICOR and LINEAR ARRAY
HPV test. J Clin Microbiol 2006;44:2428-33.
16. Stöcher M, Leb V, Holzl G, Berg J. A simple
approach to the generation of heterologous
competitive internal controls for real-time PCR
assays on the LightCycler. J Clin Virol 2002;
25:47-53.
AUGUST 2008 American Journal of Obstetrics & Gynecology
144.e6