(HPV) associated anogenital disease and potential for vaccination
advertisement

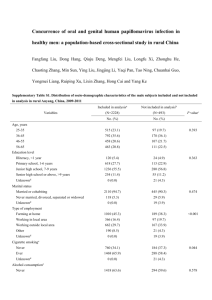
Immune control of human papillomavirus (HPV) associated anogenital disease and potential for vaccination Peter L. Stern Journal of Clinical Virology, 2005. Human papillomavirus Transmitted through sexual contact Infects the skin and mucous membranes which can lead to wart formation ~130 HPV types Associated with cervical cancer 0.25 million deaths per year 30-60% of sexually active men and women are infected with genital HPV No specific therapy available Human papillomavirus • Non-enveloped dsDNA virus • E1 and E2: minimal gene expression, suppress expression of E6 and E7 • E6: prevents cell differentiation and promotes p53 degradation • E7: prevents cell-growth arrest/differentiation • L1, L2: capsid proteins HPV infection Viral strategies to evade or subvert immune attack Keeping very low profile Non-secreting proteins No viraemia No lysis → limited antigen production HPV 16 E7 inhibits interferon regulatory factor 3 HPV 18 E6 inhibits the JAK-STAT activation response → reduced inflammatory response E5 can modulate antigen processing pathways Influence the polarization of Th cell types Immune escape as a feature of the evolution of invasive cancer HPV integrates in the genome leading to E2 inactivation which suppresses E6 and E7 transcription E6 and E7 interact with cellular tumour suppressor gene products p53 and pRb Accumulation of genetic changes and development of cancer High frequency of HLA class I down-regulation CTLs triggered after HPV integration leading to selection of immune-resistant tumour cells Prophylactic vaccines Viral capsid proteins have the intrinsic capacity to self assemble into virus-like particles (VLP) → Highly immunogenic but lacking viral DNA First trial with HPV 16 L1 VLPs induced strong immune responses and were well tolerated Peptides or recombinant proteins Peptide vaccines with HPV 16 E7 as therapy for patients with neoplasia Possible because 40% of Caucasians carry HLA-A2 allele Advantage: cost effectiveness Use of longer peptides that can be presented to CD4 and CD8 T cells driving a more vigorous CD8 CTL response Recombinant proteins have the advantage in delivery of all potential epitopes to the APC Safe but show only in a fraction of patients immunogenicity Plasmid DNA vaccines Plasmid DNA encoding antigen E6 and E7 of HPV16, 18 Encapsulation in a biodegradable polymer microparticle format potentiating the delivery to APCs Trial showed no specificity for HPV-16- or HPV-18-positive lesions Viral vector vaccines HPV vaccine vectors based on recombinant vaccinia HPV proteins are endogenously synthesized from viral DNA by host cells No restriction on patient HLA genotypes Prime-boost strategies Priming immunization (e.g. DNA plasmid or viral vector or protein) followed by a heterologous boost with a different viral vector encoding the immunogen Example: Fusion protein: HPV 16 L2E6E7 (TA-CIN) Well tolerated, induced antibody and proliferation response Induced INFγ ELISPOT response to HPV16 oncogenes Boost with TA-HPV Enhanced immunogenicity compared with either agent alone Currently available vaccines Gardasil (Merck) Based on recombinant L1 VLP Vaccination for: high risk HPV 16, 18 (cause 70% of cervical cancer) low risk HPV 6, 11 (cause 90% of genital warts) Approved June 2006 Cervarix (GlaxoSmithKline) Vaccination for: high risk HPV 16, 18