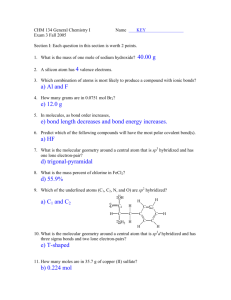
12 /+ tfB

Answer KeY
Testname:
UNTITLED1
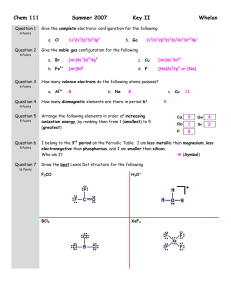
1) linear or bent
2) XeF4
3) trieonal PYramidal
4) linear
5) trigonal
PYramidal
?fr, afu' .l-
1$
t-
r',@
11) C
14)
A
15)
A
T6;]I---
1nE f 18) B
20) B
21) B
-a2lTa-@m
23) B
24)C
b
j*n f
Aipeal ar to?,t' Jq,Y
, ,/ lo s
W%,8
{t f$q,/t
, 12
/+
W tfB t p
.1P.,
-
tf
r4t, t6 tq o6
C,1 yp(
?3R,
{t /Qo' p4
gD t3
qT tE
Lrpe_sc
A4
AP@
CHEMISTRY
2O1O SCORING GUIDETINES
Ouestion 5
(8 points)
Use the information in the table below to respond to the statements and questions that follow. Your answers should be in terms of principles of molecular structure and intermolecular forces.
Compound
Ethane
Ethanol
Formula
Ethanethiol cH3cH2sH cH3cH3 cH3cH2oH
Lewis Electron-Dot Diagram
HH rt:C:e : S:H
HH
HH
H:C:C:H
HH
HH n:C:C: O: H
HH
Ethyne czHz
H;C iiC:H or
H_C
=C_H
(a) Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table above.
See the lower right cell in the table above.
One point is earned for the correct Lewis structure'
(b)Which of the four molecules contains the shortest carbon-to-carbon bond?
Explain.
Ethyne, which contains a triple bond, has the shortest
C-to-C bond. The other molecules have single
C-to-C bonds, and triple bonds are shorter than single bonds.
One point is eamed for the correct choice'
One point is earned for the correct explanation.
O 2010 The College Board.
Visit the College Board on the Web: www.collegeboard.com
AP@ CHEI\4ISTRY
2O1O SCORING GUIDELINES
Ouestion 5
(continued)
(c) A Lewis electron-dot diagram of a molecule of ethanoic acid is given below. The carbon atoms in the molecule are labeled x and y, respectively.
rQ',
H fl:e:er:Cr:fl
H
Identify the geometry of the arrangement of atoms bonded to each of the following.
(i)
Carbon.r
Trigonal planar One point is earned for the correct geometry.
(ii)
Carbon y
Distorted tetrahedral, tetrahedral or trigonal pyramidal I One point is eamed for the conect geometry.
(d) Energy is required to boil ethanol. Consider the statement "As ethanol boils, energy goes into breaking
C-C bonds,
C-H bonds, C*O bonds, and O-H bonds." Is the statement true or false? Justify your answer.
The statement is false. All of the bonds described are intramolecular; these bonds are not broken
during I
One point is eamed for the correct choice with vaporization. When ethanol boils, the added overcomes
energy I
justification.
intermolecular, not intramolecular, forces.
(e) Identiff a compound from the table above that is nonpolar. Justify your answer.
Either ethane or ethyne may be identified as nonpolar.
The ethane/ethyne molecule is nonpolar because all of the bond dipoles in the molecule cancel.
OR
The ethane/ethyne molecule is molecule is nonpolar because
symmetric. the I
I
Note: Explanation must refer to the shape of the molecule. Statements such as: "all hydrocarbons are nonpolar', 'the carbons are surrounded by hydrogens" or "there are no lone pairs" do not earn this point.
oi: point is earned for a correct choice with justification'
@ 2010 The College Board.
Visit the College Board on the Web: www.collegeboard.com
AP@ CHEMISTRY
2AIO SCORING GUIDELINES
Ouestion 5 (continued)
(f)
Ethanol is completely soluble in water, whereas ethanethiol has limited solubility in water' Account for the difference in solubilities between the two compounds in terms of intermolecular forces.
Ethanol is able to form strong hydrogen bonds with water whereas ethanethiol does not have similar capability. The formation of hydrogen bonds increases the attraction between molecules of ethanol and molecules of water, making them more soluble in each other.
Note: The answer must clearly focus on the solutesolvent interaction. Just the mention of hydrogen bonding does not earn the point.
One point is earned for the correct explanation.
@ 2010 The College Board.
Visit the College Board on the Web: wvr,nv.collegeboard.com
1) For a molecule with the formula AB2 the molecular shape is
2) The molecular geometry of
_ is square planar.
3) The molecular geometrv of the HeO+ ion is
4) The molecular qeometry of the CSr molecule is
5) The molecular geometry of the PHCI2 molecule is
_.
6) The F-B-F bond angle in the BF3 molecule is
4 Th" F-N-F bond angle in the NF3 molecule is slightly less than bondine orbitals.
-.
8) The electron-domain geometry of the AsF5- ion is octahedral. The hybrid orbitals used by the As atom for are
9) There is/are o bond(s) in the molecule below.
-
H H
:O:
llll..
.|.!-_
o--H
I
H
10) The basis of the VSEPR model of molecular bonding is
_.
A) regions of electron density on an atom will organize themselves so as to maximize s-character
B) electron domains in the valence shell of an atom will arrange themselves so as to minimize repulsions
C) atomic orbitals of the bonding atoms must overlap for a bond to form
D) regions of electron density in the valence shell of an atom will arrange themselves so as to maximize overlap
E) hybrid orbitals will form as necessary to, as closely as possiblg achieve spherical symmetry
11) According to VSEPR theory, if there are three electron domains in the valence shell of an atom, they arraneed in a(n) seometrv.
will be
A) linear
B) trigonal bipyramidal
C) trigonal planar
D) tetrahedral
E) octahedral
12) The O-C-O bond angle in the CO32- ion is approximately
A) 90" B) 120" c) 60" D) 109.5" E) 180"
A-1
13) The molecular geometry of the BrO3- ion is
A) tetrahedral
B) trigonal pyramidal
C) T-shaped
D) trigonal planar
E) bent
14) The bond angles marked a, b, and c in the molecule below are about respectively.
:O: H :O: lllll..
iriYc'rj-'o'-H
HPH
, and
A) 109.5" 120",109.5o
B) 109.5" 90",120 c) 120" 120",94"
D) 90" 90o, 90o
E) 120",120",109.5"
15) Of the molecules below, only
A) BFs
.-*..-.._- is nonpolar.
B) BrCl3 C) PBrs D) NF3 E) IFs
16)Themo1eculargeometryofthePF3mo1eculeis'andthismoleculeis-'
A) trigonal planar, nonPolar
B) tetrahedraf unipolar
C) trigonal pyramidaf nonPolar
D) trigonal pyramidaf
Polar
E) trigonal planar, Polar l7)Theelectron-domain geometry of a carbon-centered compound is tetrahedral. The hybridization of the central carbon atom is
A) st'd
B) sp
C) sP342
D) tP2 s) tP3
18)ThehybridizationsofnitrogeninNF3andNH3are-and.''respectively.
A) sp, sp3
B) sp3, sp3
C) sp2, sp2
D) sp2, sp3
E) st'' sp
19) A typical double bond
.-...-_.-.
A) is stronger and shorter than a single bond
B) imparts rigidity to a molecule
C) consists of traro shared electron pairs
D) consists of one o bond and one n bond
E) All of the above answers are co$ect.
A-2
20) Which of the following molecules or ions will exhibit delocalized bonding?
Nq- NH4+
N3-
A) NH4+ and N3-
B) NO2- only q Nq-,
NH4+, and N3-
D) N3- only
E)
NQ- andN3-
21) The hybridization of the carbon atom labeled x in the molecule below is
A)
sp
H
HH:O:X
I I ll
//.-
I
H_C_H
I
H
B)
s/
C)
sp3
D)
sp36
E) sp362
22)The more unpaired electrons in a species, the stronger is the force of magnetic attraction. This is called
23)UsingtheVSEPRmodel,themoleculargeometryofthecentralatominPF5is-
A) square planar
B) trigonal bipyramidal
C) seesaw
D) tetrahedral
E) square pyramidal
24)The hybrid orbital set used by the central atom in NOg- is
A) sdd2
B) sd
C) sp2
--*.
D)
"dd D sp
A-3
r
-., t
'' .:.-
:-.-. : I
- your responses appropriate. to these questions
Explanations should be ciear and will be scored on the basis of the accuracy and relevance of the information citedwell orgamzed.Examples and equations
Specific answers are preferable to broad, diffuse responses. rnay be included in your respon$es where t'
!fr.
' Use the infonrration in the table below to respond to the statements and questions that should be in terms of principles of molecular structure and intermolecular forces.
follow' Your answers
Compound
Ethanethiol
Ethane
Ethanol
Formula cH3cH2sH cH3cH3 cH3cH20H
Lewis Electron-Dot Diagram
HH n:e te : S;H
HH
HH
II:C: C:tt
HH
HH
H:e:e:b:H
HH
Ethyne czllz
9p
\outr
Paftbr
(a)DrawthecompleteLewiselectron.dotdiagramforethyne.iru*e-aFpr6pEiat@.
(b) Which of the four molecules contains the shortest carbon-to-carbon bond? Explain.
(c) A Lewis electron-dot diagram of a molecule of ethanoic acid is given below. The carbon atoms in the molecule are labeled x and y, respectively.
t9,t
H
U
,9 t C,, d.rt H
H
Identify the geometry of the arrangement of atoms bonded to each of the following.
(i)
Carbon r
(ii)
Carbon y
(d) Energy is required to boil ethanol. Consider the statement
"As ethanol boils, energy goes into breaking C-C bonds,
C-H bonds,
C-O bonds, and
O-H bonds." Is the statement true or false? Justify your answer.
(e) Identify a compound from the table above that is nonpolar. Justify your answer.
(f)
Ethanol is completely soluble in water, whereas ethanethiol has limited solubility in water" Account for the difference in solubilities between the two compounds in terms of intermolecular forces'
,:a: :
-r':#
*€f; eL;
1) The electron-domain geometry of the AsF6- ion is octahedral. The hybrid orbitals used by the As atom for bondine are orbitals.
2) The molecular geometry of is square planar.
3) The molecular geometry of the BtO3- ion is
A) trigonal pyramidal
B) T-shaped
C) trigonal planar
D) bent
E) tetrahedral
-
-
4) For a molecule with the formula AB2 the molecular shape is
5) The F-N-F bond angle in the NF3 molecule is slightly less than
6) The hybrid orbital set used by the central atom in SO2 is
A) sp36
B) sp342 I sp
7) The molecular geometry of the PF3 molecule is
A) trigonal pyramidaf nonpolar
B) trigonal planar, nonpolar
C) trigonal planar, polar
D) tetrahedral, unipolar
E) trigonal pyramidaf polar
-
D) rp3 and this molecule is
8) A trrpical double bond
A) consists of one cr bond and one n bond
B) is stronger and shorter than a single bond
C) imparts tigtdity to a molecule
D) consists of tlt'o shared electron pairs
E) All of the above answers are correct.
q'1
9) The O-C-O bond angle in the CO32- ion is approximately
A)
109.5'
B)
120'
C)
180"
D)
90'
10)Thehybridizationofthecarbonatomlabeledxinthemoleculebelowis-.
A)
sp
HH:O:X
I I
ll
//..
"r"
H-C-H
I
H
B) sp2 C) sp3 D)
sp34
E) 60' g sp342
11) The more unpaired electrons in a species, the stronger is the force of magnetic attraction. This is called
B-1
o bond(s) in the molecule below'
L2) There is/are
-
H
I t:-_
H
:O:
lll..
l"
c---+I
I
H
13) The molecular geometry of the PHCI2 molecule is
14) The electron-domain geometry o{ a carbon-centered compound is tetrahedral. The hybridization of the cerrtral carbon atom is
A) sp --.
B) sP2
C) sP3
D) sp3d E) sp3d2
15)ThehybridizationsofnitrogeninNF3andNH3are-and,respectively.
A) rp, sp3 B) rt', tp3 C)
tf,
tp3 D) sp3, sp E) sf, sp2
16) 1/hich of the following molecules or ions will exhibit delocalized bonding?
NOZ- NH4+
N3-
A) NH4+ and
N3-
B) NO2- only
C) NO2-, NH4+, and N3-
D) N3- only
E) NO2- andN3-
17) The molectrlar geometry of the H3O+ ion is
18) Of the molecules below, oniy
A)
BrCl3
B)
BF3 is --'
C) PBr3
D) NF3 E) IFg
19) The basis of the VSEPR model - of molecular bonding is
A) electron domains in the valence shell of an atom
----.
will arrange themselves so as to minimize repulsions
B) regions of electron density on an atom will organize *remselves so as to maximize s-character
C) hybrid orbitals will form as necessary io, as closely as possiblq achieve spherical symmetry
D) regions of electron density in the valence shell of an atom will arrange thernselves so as to maximize overlap
E) atomic orbitals of the bonding atoms must overlap for a bond to form
B-2
20) According to VSEPR theory arranged in a(n) if there are three electron domains in the valence shell of an atom, they will be geometry.
A) linear
B) trigonal planar
C) trigonal bipyramidal
D) octahedral
E) tetrahedral
21) The F-B-F bond angle in the BF3 molecule is
_.
22)1"le bond angles marked a, b, and c in the molecule below are about respectively.
:o: H
:o:
..lllll..
:-rTYffi-'e-H
HUH
A) 120" 120o,90"
B) 90o, 90",90" c) 120o,I20",709.5"
D) 109.5", 120o,109.5o
E) 109.5.,90",720o
. and
23) Using the vsEPR model the molecular geometry of the central atom in pF5 is _
A) square pyramidal
B) square planar
C) seesaw
D) tetrahedral
E) trigonal bipyramidal
24)The molecular geometry of the CS2 molecule is _.
B-3
ffi
Compound
Ethane
Ethanol
Fomrula
Ethanethiol cH3cH2sH cH3cH3 cH3cH2oH
Lewis Electron-Dot Diagram
HH n:e:e:3:H
HH
HH
H:C:e;n
HH
HH fr;e :C:O:H
HH
Ethyne c$z
9p
\CIur
wib{
(a) Draw the complete Lewis electron-dot diagram for
ethynei
v-e.
(b)
(c)
Which of the four molecules contains the shortest carbon-to-carbon bond?
-<
Explain.
A Lewis electon-dot diagram of a molecule of ethanoic acid is given below. The carbon atoms in the molecule are labeled x and y, respectively
..'9'
H
H:O:C":Cr:11
H
Identify the geometry of the arangement of atoms bonded to each of the following.
(i)
Carbon
r
(ii)
Carbon y
(d) Energy is required to boil ethanol. Consider the statement "As ethanol boils, energy goes into breaking C-C bonds,C-H bonds,
C-O bonds, aud O-H bonds.o' Is the statement true or false? Justiff your answer.
(e) Identify a compound from the table above that is nonpolar. Justify your answef.
(0 Ethanol is completely soluble in water, whereas ethanethiol has limited solubility in water. Account for tbe difference in solubilities between the two compounds in terms of intermolecular forces.






![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)