Chapter 8
advertisement

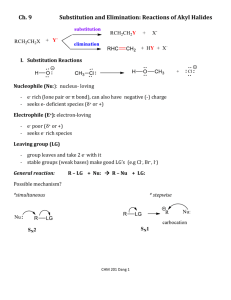
Chapter 8 – 5th ed CHEM 321 Organic Chemistry I - Professor Kathleen Kilway "Organic Chemistry" by Maitland Jones, 5th edition Chapter 8 – 1, 2, 3, 5, 7, 8, 15, 20, 21, 22, 24, 26, 27, 28. CHAPTER 8: Elimination Reactions: The E1 and E2 Reactions Section 8.1 I. Preview A- Elimination versus Substitution Section 8.2 II. The Unimolecular Elimination Reaction: E1 A- Rate Law 1- Rate determining step is ionization of substrate. a- Same as for SN1, Rate (v) = k [R-L]. b- First order reaction. c- First step yields carbocation intermediate (same as in SN1 reaction). 2- Instead of adding a nucleophile as is done during the second step of a SN1 reaction, the substrate is deprotonated. An alkene is formed. a- The nucleophile is acting as Brønsted base. Figure 1 Ch 8 - 1 Chapter 8 – 5th ed 3- Mixture of E1 and SN1 products depends on nucleophile’s basicity. a- Strong Brønsted bases - E1. b- Good nucleophiles favor SN1. B- Selectivity in the E1 Reaction 1- More stable alkene is favored, thus more substituted. 2- Saytzeff elimination - produces the most substituted alkene possible [versus Hofmann elimination - formation of the less stable alkene]. 3- Competes with the SN1 reaction. 4- See Figure 8.3 on page 334 for an example. C- Dehydration Section 8.3 III. The Bimolecular Elimination Reaction: E2 A- Rate Law 1- Second order process, dependent on substrate and nucleophile. 2- Rate (v) = k [R-L] [Nu-] Figure 2 3- Strong base attacks proton attached to β-carbon (carbon adjacent to the carbon bearing the leaving group). 4- E2 competes with SN2. Ch 8 - 2 Chapter 8 – 5th ed B- Effect of Substrate Branching on the E2-SN2 Mix 1- The more branching of substrate, the more likely reaction will proceed E2. 2- Tertiary substrates only undergo E2 reactions under E2-SN2 conditions. C- Stereochemistry of the E2 Reaction 12345- Only syn (0 °) and anti (180 °) arrangements of substrate can lead to π-bond formation. Syn elimination - elimination where the dihedral angle between C-H and C-L is 0 °. Anti-elimination - elimination where the dihedral angle between C-H and C-L is 180 °. Anti elimination is favored over syn elimination (See Figure 8.15 on page 340). Formation of the more substituted alkene is also favored. Figure 3 D- Selectivity in the E2 Reaction 1- The larger the size of the base the more likely 1:1 (Hofmann/ Saytzeff) product ratio. 2- Regiochemistry - refers to the outcome of the reaction in which more than one orientation of substituents is possible in the product. 3- Hofmann elimination - formation of the less stable alkene and is favored when: a- The leaving group is fluoride, ammonium (R3N+), and sulfonium (R2S+). b- The base is a strong one like an alkoxide (RO-). Ch 8 - 3 Chapter 8 – 5th ed c- For R3N and R2S as leaving groups, the Hofmann product formed is due to a steric effect. d- See Figure 8.19 on page 343 for an example. + + Figure 4 Figure 4 (Also see figure 8.23 on page 348). Ch 8 - 4 Chapter 8 – 5th ed Section 8.4 IV. Transition States: Thermodynamics versus Kinetics Energy Barriers in Chemical Reactions: The Transition State and Activation Energy 1-It is the difference in free energy between the starting material and the transition state in a reaction. 2-This energy must be supplied in order for a reaction to occur. 3-Note that we now use ∆G‡to denote activation energy and not Ea 4-SN2-For the forward reaction: a- also see figures 8.16 and 8.17 on page 344. Figure 2-For the reverse reaction: Figure A- Thermodynamics 1-Thermodynamics is the study of energy relationships. B- Kinetics 1-This is the determination of the rates of reaction. C- Thermodynamic Control 1-A thermodynamically controlled reaction is a reaction in which the product is Ch 8 - 5 Chapter 8 – 5th ed determined by the relative energies of the products. D- Kinetic Control 1-A kinetically controlled reaction is a reaction in which the product distribution is determined by the heights of the different transition states leading to products. E- Hammond Postulate 1-The transition state for an endothermic reaction will resemble the product. 2-It can be equivalently stated as, "The transition state for an exothermic reaction will resemble the starting material." Section 8.5 V. Rearrangements of Carbocations A- Hydride and Methyl Shifts 1-Only happen when going through carbocation. 2-Therefore, cannot happen in SN2 and E2 reactions. 3- It is in the case when there is a secondary carbocation, which can undergo one of these shifts to make it tertiary. 4- Need to know that it only happens once. 5- Shifts hydride first (if available), then a methyl (second choice) but notehr larger than a methyl group. Section 8.6 VI. Special Topic: Other Eliminations A- Thermal syn Eliminations – when no other option is available B- Eliminations through loss of small molecules Section 8.7 VII. Special Topic: Enzymes and Reaction Rates A- Enzymes 1-Enzymes act as catalysts to lower the activation energy, thereby increasing reaction rates in biological systems, at a much lower heat than would be required in the laboratory. Ch 8 - 6 Chapter 8 – 5th ed Section 8.8 VIII. Special Topic: Why Are Rearrangements of Carbocations Fast? Summary of Substitution versus Elimination A- SN1 Reaction competes with E1 Reaction (3o and some 2o Carbons) but Nu is neutral (e.g., H2O or HOR) B- SN2 Reaction (THF – good solvent- nonpolar) competes with E2 Reaction (2o, 1o, and methyl Carbons) – Nu must be charged (e.g., NaOH/H2O or NaOR/HOR). C- Exceptions are: Substitution only for azide and cyanide E2 only when tertiary R-L and charged Nu () Tert-butoxide (CH3)3CO- only favors E2 for secondary and tertiary R-L Combination of charged Nu and LG (sulfonium, ammonium, or fluoride) – more stable alkene is minor; less stable alkene is major. SN1/E1 – can have hydride and methyl shifts for a secondary carbocation D- In order to decide which reaction is favored, follow the following flow chart: 1- Is the R of the alkyl halide methyl (a), primary (b), secondary (c), or tertiary (d)? 2- Is the nucleophile a good base? Yes or no? 3- Is the solvent polar? Yes or no? E-­‐ Flow Chart (a) methyl carbon, no - not a good base, no - not a polar solvent = SN2 only SN2 possible (b), yes, no = E2 (b), no, no = SN2 (c), yes, no = E2 (c), no, no = SN2 (c), no, yes = SN1 (d), yes, yes = E1 (d), no, yes = SN1 Ch 8 - 7 Chapter 8 – 5th ed Section 8.9 IX. Summary - Vocabulary Equilibrium constant Gibbs free energy Thermodynamic vs. Kinetic Control Activation Energy Entropy Enthalpy Dependence of rate on temperature, pressure, and solvent Transition States Microscopic reversibility Hammond Postulate Ch 8 - 8 SN1, SN2, E1, or E2 R-L: where R contains C such as CH3; CH3-L is methyl; CH3CH2-L is 1o; (CH3)2CH-L is 2o; (CH3)3C-L is 3o L is a leaving group such as a halide or F-, Cl-, Br-, or I- (best LG is I-) Nu: can be neutral such as H2O or ROH or charged such as NaOH or NaOR this can add to C (SN1 or SN2) or act as a base to deprotonate (E1 or E2) R= methyl or 1o SN2 only R = 2o Nu = -:CN: or -:N3 (e.g., NaCN or NaN3) SN2 and SN1 R = 3o SN1 only R= methyl or 1o no reaction Nu = neutral H2O or ROH R = 2o and 3o SN1 and E1; for 2o hydride/methyl shifts may occur R= methyl Nu = charged such as NaOH or NaOR R= 2o R= 3o or SN2 only 1o SN2 and E2 E2 only For Elimination Reactions, normally more/most substituted alkene - major product(s) (Saytzeff versus least stable Hofmann products) H H H > H > H> H H 4 sp2-sp3 or 4C - 0H = 4 3 sp2-sp3 or 4C - 1H = 3 > H H H 2 sp2-sp3 3 sp2-sp3 or 4C - 2H = 2 or 4C - 3H = 1 0 sp2-sp3 or 4C - 4H = 0 Hoff - major product when Nu: charged such as NaOH or NaOR (E2) and L = sulfonium (SR2), ammonium (NR3), or F S(CH3)2 Lookin down C2 and C3, must have Hb anti to have E2 so Hb has to be anti S(CH3)2 but gauche interaction is not good to have Saytzeff product H Hb Ha Hb Ha S(CH3)2 Ha H Ha Looking down C1 and C2, must have anti to have E2 so Ha has to be anti which is possible with 3 Ha