Name
advertisement

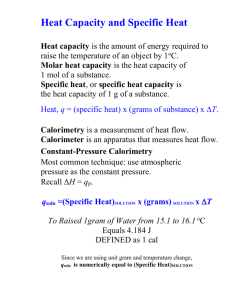
Name:_______________________________ Date:_________________ Hr:____ AP CHEMISTRY Calorimetry LAB Investigating the Energy Content of Foods INTRODUCTION: Food supplies energy for all animals—without it we could not live. The quantity of energy stored in food is of great interest to humans. The energy your body needs for running, talking, and thinking comes from the foods you eat. Not all foods contain the same amount of energy, nor are all foods equally nutritious for you. An average person should consume a minimum of 2,000 kilocalories per day. That is equivalent to 8,360 kilojoules. Calories and joules are both units of energy. We will use joules in this experiment since it is the SI unit for energy. You can determine energy content of food by simply burning a portion of it and capturing the heat released to a known amount of water. This technique is called calorimetry. The energy content of the food is the amount of heat produced by the combustion of 1 gram of the food, and is measured in kilojoules per gram (kJ/g). In this investigation, you will determine the energy content of food samples. You will use the energy from a burning food sample to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it (in kJ), using the formula q = mcΔT where q is heat, c is the specific heat capacity of water (4.18 J/g°C), m is the mass of water, and ΔT is the change in temperature of the water. Finally, the amount of the food samplse burned will be taken into account by calculating the heat per gram of the food sample consumed in the combustion. The following resources may be helpful in completing the analysis of your experimental data. What’s in the Food You Eat Search Tool, United States Department of Agriculture, http://reedir.arsnet.usda.gov/codesearchwebapp/(wlrrek55vb2mdb45zgo1jj55)/codesearch.aspx (short link tinyurl.com/usdafoodsearch) What’s in the Food You Eat, United States Department of Agriculture, http://www.ars.usda.gov/Services/docs.htm?docid=7783 (short link tinyurl.com/usdafooddoc) FOOD ALERGY WARNING: While you will not be eating any of the food samples during the investigation, they will be present and they will be burning during the experiment. If you have severe food allergies, please inform your instructor prior to the experiment, so precautions may be taken and alternative food samples may be obtained. Page 1 of 5 2013 Name:_______________________________ Date:_________________ Hr:____ AP CHEMISTRY Calorimetry LAB PreLab Guiding Questions/Simulations: *completed on a separate sheet of paper 1. Using the information available from the USDA, what is the energy content (in kJ) per gram of each of the following foods? Show your work if calculations are necessary. a. Banana chips d. Microwave buttered popcorn b. Corn flakes e. Regular potato chips c. Marshmallows f. Roasted, salted peanuts 2. A 1.25-g food sample was completely combusted to heat a 100.00-g sample of water, with an initial temperature of 22.5 °C, to a final temperature of 52.4 °C. Determine the energy content of the food, in kilojoules per gram. INVESTIGATION: Procedure: Connect the temperature probe to the data-collection interface. Following your instructor’s directions set up the data-collection program to collect data for twenty minutes. Obtain a food sample and a food holder. CAUTION: Do not eat or drink in the laboratory! Record the identity of the food sample and the combined mass of the sample and the food holder. Record the mass of an empty can. Add about 50-mL of chilled water to the can and record the combined mass of the can and water. Set up the apparatus as shown in the figure at right. Use an iron ring and stirring rod to suspend the can about 2.5 cm (~1 inch) above the food sample. Use a utility clamp to suspend the Temperature Probe in the water without touching the sides or bottom of the can. Important: Stir the water until the Temperature Probe has cooled to the temperature of the water before proceeding with further data collection. Begin the data-collection program. Record the initial water temperature. Remove the food sample and its food holder from under the can and use a wooden splint to light it. Quickly place the burning food sample directly under the center of the can. Allow the water to be heated until the food sample stops burning. CAUTION: Keep hair and clothing away from an open flame. Stir the water until the temperature stops rising. Record this final temperature, and then stop the data-collection program. Allow the burned food sample to cool for about a minute, then record the final mass of the food sample and the food holder. Repeat the procedure for a second sample. When data collection is complete, have your instructor initial your data. Follow your instructor’s directions for waste disposal. Page 2 of 5 2013 Name:_______________________________ Date:_________________ Hr:____ AP CHEMISTRY Calorimetry LAB Investigating the Energy Content of Foods Data and Observations: LAB PARTNER:__________________ Data Table 1: Calorimetry of Food Samples by Combustion ___________________ Sample ___________________ Initial sample and holder mass (g) Empty can mass (g) Can and water mass (g) Initial water temperature (°C) Final water temperature (°C) Final sample and holder mass (g) Other observations: Instructor’s Initials:_____ Calculations: 1. Using data from one of your samples, show your work for the calculation of the energy content of the food, in kJ/g. 2. Calculate the energy content of the other sample and record it here: __________ Page 3 of 5 2013 Name:_______________________________ Date:_________________ Hr:____ AP CHEMISTRY Calorimetry LAB Questions: 1. Calculate the percent efficiency of the experiment by dividing your experimental value by the accepted value, and multiply by 100. Do this for each food sample. Accepted values for the energy content of food samples are available from the USDA (see “Introduction” and “PreLab Guiding Questions/Simulations”). 2. Discuss heat loss factors that contribute to the inefficiency of the experiment. Focus on experimental sources of error and not your mistakes. 3. If the procedure were modified so that the precise volume of water used were known instead of the can’s mass with and without the water, what assumption(s) would have to be made in the lab in order to determine the energy content of the food? How would this influence the results? Page 4 of 5 2013 Name:_______________________________ Date:_________________ Hr:____ AP CHEMISTRY Calorimetry LAB 4. For each of the following sources of error, predict the effect (increases, decreases, or no effect) on the experimentally determined energy content of the food. Justify each response. a. Your lab partner ate a piece of the food sample before determining its mass with the food holder. Your lab partner also received a detention and was no longer permitted in lab. b. Instead of adding 50 mL of water to the can, 100 mL of water was used. c. After igniting the food sample with the wood splint, your lab partner burnt his/her finger and cried about it for a minute before placing the food sample under the can. d. Soot developed on the bottom of the can during the combustion of the first food sample, but it was not wiped off prior to massing the empty can for the second food sample trial. 5. This experiment was performed again using a paraffin wax candle to determine the energy content of paraffin wax. The candle mass changed from 11.38 g to 10.81 g as the 100.38-g water sample’s temperature changed from 4.9 °C to 41.3 °C. Determine the heat of combustion (in kJ/mol) of paraffin (C55H52) from this experimental data? Page 5 of 5 2013