Key Concepts in Acute Pain Management
advertisement

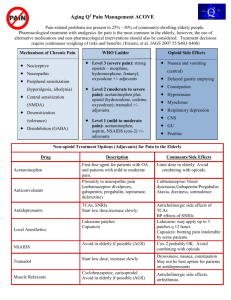
KEY CONCEPTS IN ACUTE PAIN MANAGEMENT PGY-1 Faculty of Medicine University of Ottawa Feb.18, 2009 John Penning MD FRCPC Director Acute Pain Service The Ottawa Hospital Objectives General Key Concepts – The “real cost” of acute pain – Multi-modal analgesia – New Dimensions in pain management – How and when to use naloxone Objectives Discuss key concepts of each modality – COX-inhibitor as Foundational analgesic – Tylenol # 3 has it’s limitations – Opioids? – think outside the “box” – Tramacet – a “me too” drug? Or something new to add? – Anti-pronociceptive agents for difficult acute pain Consequences of poorly managed acute post-operative pain The Patient suffers – – – – – – CVS: MI, dysrhythmias Resp: atelectasis, pneumonia GI: ileus, anastamosis failure Endocrine: “stress hormones” Hypercoagulable state: DVT, PE Impaired immunological state • Infection, cancer, wound healing – Psychological: • Anxiety, Depression, Fatigue, Sleep – Chronic Post-surgery/trauma Pain Deprivation Consequences of poorly managed acute post-operative pain The Hospital – – – – Increased costs $$$ Poor staff morale Reputation/Standing in the Community, Nationally Accreditation • Canadian Council on Health Services Accreditation; Acute Care Standard 7.4 2005. • TOH Pain Management Council 2006 • TOH Pain Assessment and Management Policy ADM 8 – Litigation Consequences of poorly managed acute post-operative pain The Healthcare professional – Morale – Complaints to College – Litigation Risk Factors for severe post-op pain While incision size and type of surgery are predictors of post-op pain, the greatest source of variability is the PATIENT. – Younger age – Female – Pre-operative pain issues – Anxiety, depression, catastrophizing Chronic Post-Surgical Pain Risk Factors Brandsborg B. et al. Risk factors for chronic pain after hysterectomy. Anesthesiology 2007; 106: 1003 – 12. – Pre-operative pelvic pain as the main indication for surgery – Pain problems elsewhere – Previous C/S Procedure done with spinal anesthesia shown to decrease incidence of CPSP. The New Challenges in Managing Acute Pain after Surgery and Trauma Patients/Society more “aware” of their rights to have good pain control – We are being held accountable Pressure from hospital to minimize length of stay – Control pain, yet limit the side-effect burden and complications secondary to opioids The New Challenges in Managing Acute Pain after Surgery and Trauma The Opioid Tolerant Patient – The greatest change in practice/attitudes in the last 10 years is the now wide spread acceptance of the use of opioids for CHRONIC NON-MALIGNANT PAIN – Renders the “usual” standard “box” orders totally inadequate in these patients Get an accurate Pain/Analgesic History – The Brief Pain Inventory – “BPI” Brief Pain Inventory: Charles Cleeland What is the “Best Way” to manage Acute Pain? FIRST, DO NO HARM Therefore, the “best way” is a BALANCE Patient Safety Effective Analgesic Modalities Analgesia with Opioids alone The harder we “push” with single mode analgesia, the greater the degree of side-effects Side-effects Analgesia Case Problem: Severe Respiratory Depression after Ketorolac? Healthy 34 yr. patient c/o severe incisional pain in PACU after ovarian cystecomy Received 200 g fentanyl with induction and 10 mg morphine during case, no foundational analgesic given PCA morphine started in PACU, plus nurse supplements totaled 26 mg in 90 minutes Still c/o pain, 30 mg ketorolac IV, given with some relief after 15 minutes, so patient sent to ward 60 minutes later found unresponsive, cyanotic, RR 4/min. Case Problem: Severe Respiratory Depression after Ketorolac? Pharmacodynamic drug interaction between morphine and NSAID – morphine’s respiratory depressant effect opposed by the stimulatory effects of pain, busy PACU environment – NSAID decreases pain, morphine’s effect unappossed Case Problem: Severe Respiratory Depression after Ketorolac? Safer approach? – Add NSAID foundational analgesic ASAP – Gain control of acute pain with fast onset, short acting opioid(fentanyl) In a patient previously “loaded” with opioids and c/o pain despite some sedation, Monitor closely for oversedation and respiratory depression after pain is alleviated by any means! The problem with the “Little Pain – Little Gun”, “Big Pain – Big Gun” Approach With opioids analgesic efficacy is limited by side-effects “Optimal” analgesia is often difficult to titrate – 10 – fold variability in opioid dose : response for analgesia – A dose of opioid that is inadequate for patient A can lead to significant S/E or even death in patient B. • Many patient factors add to the difficulty – Opioid tolerance, anxiety, obstructive sleep apnea, sleep deprivation, concomitantly administered sedative drugs Multi-modal Analgesia “With the multimodal analgesic approach there is additive or even synergistic analgesia, while the sideeffects profiles are different and of small degree.” Side-effects Analgesia Pain Pathways There is as of yet no single silver bullet!! Acute Pain Management Modalities Cyclo-oxygenase inhibitors – Non-specific COX inhibitors(classical NSAIDs) – Selective COX-2 inhibitors, the “coxibs” – Acetaminophen is probably COX-3 Local anesthetics Opioids NMDA antagonists – Ketamine, dextromethorphan Anti-convulsants – Gabapentin, Pregabalin NSAID, Coxibs and Acetaminophen CONCEPT # 1 The foundation of all acute pain Rx protocols. ”First on last off” sole agent in mild /moderate pain Analgesic efficacy is limited inherently In contrast, with opioids efficacy is limited by S/E Opioids added as required opioid sparing effect 30-60 % COX-INHIBITORS vs. OPIOIDS Efficacy Limited Inherently Inter-patient dose variability Small Limited by S/E Large, making dose titration difficult Life threatening complications Upper GI bleeding With chronic use Resp. depression Risk is early COX-INHIBITORS vs. OPIOIDS Toxicity, S/E Tissue/organ toxic Drug tolerance Not evident neurologic dysfunc tolerance is part of normal response Abuse potential Nil Yes Cyclo-oxygenase inhibitors Acetaminophen Naproxen Celecoxib Ketorolac Numerous others Cell Membrane Phospholipids Phospholipase Arachidonic Acid COX-1 COX-2 Prostaglandins Prostaglandins Gastric Protection Platelet Hemostasis Acute Pain Inflammation Fever Why a COX-2 inhibitor? No effects on platelets! Better GI tolerability – Less dyspepsia, less N/V Equivalent analgesic efficacy with nonselective COX-inhibitors Celecoxib and “sulfa allergy” Allergy to sulfa?? History, Please! – Most reported allergies are bogus: N/V, diarrhea – A rash with sulfonamide anti-biotics? Celecoxib belongs to the “other” class of sulfonamides: furosemide, glyberide, etc. – Do not use celecoxib is history of anaphylaxis or severe cutaneous reaction (Steven-Johnson sydrome. etc.) with a sulfonamide Two hours before surgery associated with post-op pain 1. Celecoxib 400 mg PO If severe allergy to sulfa? 2. OR Naproxen 500 mg PO Contra-indications to NSAID? Plus Acetaminophen 1000 mg PO Contra-indications to Celecoxib/NSAIDs Patients with the “ASA triad” – Risk of severe asthma, angioedema precipitated with COX-inhibitor Renal insufficiency or risk there of – especially if risk of hypovolemia periop – Patient on ACE inhibitors or ARBs – Vascular patients having aortic cross-clamp and/or probable angiogram peri-operatively Poorly controlled hypertension – Especially if pt. is on ACE inhibitor, potent loop diuretics Contra-indications to Celecoxib/NSAIDs Congestive heart failure – Fluid/sodium retention Active peptic ulcer disease The Opioids We have to stop trying to put every patient in the “analgesic dose box” Meperidine 75 mg IM Q4H prn Tylenol #3 1 – 2 PO Q4H prn Opioids CONCEPT # 2 Pharmacokinetic + Pharmacodynamic patient to patient variability results in 1000 % variability in opioid dose requirements (standardized procedure, opioid naïve patient) – opioid dosage must be individualized – therefore, if parenteral therapy indicated, IV PCA much better suited to individual patient needs than IM/SC True or False? One opioid is just like any other, in terms of analgesic efficacy and side-effects. Opioids – Are they all the same? Morphine Hydromorphone (dilaudid) Fentanyl Oxycodone (parenteral n/a) Meperidine (demerol) Opioids – Do they all act the same? Opioids work as analgesics by activating endogenous inhibitory pain modulating systems Opioid receptors – Mu, Delta and Kappa – Large genetic variability in expression Good choice in one patient may be poor choice in another – Analgesic efficacy – Side-effect profile Meperidine Morphine Atropine Fentanyl Bupivacaine Meperidine’s major problem Normeperidine – The “ugly” metabolite • Neuroexcitatory: twitches, dilated pupils, hallucinations, hyperactive DTR, seizures • Non-opioid receptor mediated, no tolerance • Half-life is 15 – 20 hours N-demethylation True or False? One opioid is just like any other, in terms of analgesic efficacy and side-effects. Answer. There is considerable variability between patients in response to different opioids. Opioid Myths that still prevail! Codeine is a “weak” opioid? Codeine is inherently safer than the more potent opioids? CODEINE – A drug whose time has come and gone? N Engl J Med 351; 27 Dec. 30, 2004 Problems with Codeine 62 yr. male with CLL, presents with bilateral pneumonia. Broncho-lavage revealed yeast – Anti-biotics: Ceftriaxone, clarithromycin, voriconazole – Codeine 25 mg PO TID for cough Problems with Codeine Day 4 became markedly sedated, pinpoint pupils and ABG reveals PaCO2 of 80 mmHg. Marked improvement with Naloxone. What’s the expected morphine blood level? Answer: 1 to 4 mcg/L This patient’s morphine blood level? – 80 mcg/L Codeine Metabolism in Normal Circumstances The major pathways convert codeine to inactive metabolites – CYP3A4 pathway yields norcodeine – Glucuronidation The minor pathway, about 10%, yields morphine – CYP2D6, essential for analgesic effect 60 mg Codeine PO – approx. 4 mg morphine SC Variability! 60 mg PO Codeine yields potentially 0 to 60 mg parenteral morphine Genetic Variability And drug interactions 1% Finland 10% Greek 30% East Africa Potential Codeine Drug Interactions Major pathway – CYP3A4 – Inducers decrease codeine effect – Inhibitors increase codeine effect Minor pathway - CYP2D6 – Inducers increase codeine effect – Inhibitors decrease codeine effect Inhibitors of CYP2D6 SSRIs (potent) especially PAXIL Cimetidine, Ranitidine Desipramine Propranolol Quinidine (potent) Viagra Many anti-biotics and chemo Why not just go with Percocet? Too potent for some patients – 5 mg oxycodone = 60 mg codeine It too, may be a pro-drug? – Codeine is to Morphine as – Oxycodone is to ?? Oxymorphone – The jury is still out on this one Instead of Tylenol # 3 ? Acetaminophen 650 mg PO Q4H with Morphine 10 – 20 mg PO Q4H prn OR Dilaudid 2 – 4 mg PO Q4H prn Newly available Tramacet 1 – 2 tabs PO Q4H prn Opioids STOP Hydromorphine 1 – 4 mg PO/IM/IV Q4H prn NOT! This represents up to 30 fold range in peak effect in any given patient 1 mg PO ---- 4 mg IV bolus homeopathic dose ---- potentially lethal Opioids: Rational multi-route orders? Foundation of Acetaminophen/NSAID Morphine 5 - 10 mg PO Q4h prn Morphine 2.5 - 5 mg s.c. Q4h prn Morphine 1-2 mg IV bolus Q1h prn Hydromorphone 1 - 2 mg PO Q4h prn Hydromorphone 0.5 – 1 mg s.c Q4h prn Hydromorphone 0.25 – 0.5 mg IV Q1h prn TRAMADOL What about Tramacet? Combination drug, 325 mg of acetaminophen + 37.5 mg of tramadol Ordered like T#3 – 1 to 2 tabs Q4H prn Efficacy limited by max dose for acetaminophen. Opioids can be added as required! Is Tramadol New? Just recently available in Canada, as Tramacet Synthesized in 1962, available in Germany since 1977, UK 94, US 95 where IV formulation is also available Minimal risk of respiratory depression and abuse potential, never been a “scheduled” drug Now #1 prescribed centrally acting analgesic worldwide > 50 million patients Tramacet - How does it work? Inherent multimodal action – 4 distinct mechanisms 1. acetaminophen 2. Weak mu agonist – very weak opioid 3. Augments endogenous anti-nociceptive modulation via serotonin 4. and norepinephrine pathways Advantages of Tramacet? Tramadol’s “strength” lies in it’s “weakness” as an opioid – Poor Mu receptor affinity Minimal opioid effect – Less constipation, faster return to normal bowel function – Less N/V – No sig. respiratory depression – No sig. risk for abuse (not classified as narcotic) Advantages of Tramacet? Tramadol’s “strength” lies in it’s “weakness” as an opioid – Poor Mu receptor affinity Tramadol does not antagonize the action of classic mu agonists like morphine, dilaudid or fentanyl – Unlike the partial agonist/antagonists such as Talwin, Nubain, Stadol Other mu agonist may be added Does Tramacet work? Combination tramadol plus acetaminophen for postsurgical pain. Adam B. Smith et al. The American Journal of Surgery 2004; V187: 521 – 527. 1 tab of Tramacet = 1 tab T #3 – IN YOUR AVERAGE PATIENT !! Tramacet Precautions Liver Toxicity – Risk of acetaminophen dose exceeding recommended 4 gm/day in 70 kg patient, if patient inadvertently takes other acetaminophen products, especially OTC. Why combination analgesics are not a great idea Acetaminophen-Induced Acute Liver Failure: Results of a USA Multicenter, Prospective Study. Hepatology, Vol. 42, No. 6, 2005. Larson et al. 22 centers, 662 cases ’98 – ’03. 50% cases due to acetaminophen 50% of acetaminophen cases inadvertent Tramacet Precautions Risk of seizures, very rare – U.K. Safety Committee reports 1:7000 – Most cases involving interaction with proconvulsant agents or large IV doses of tramadol – Risk taking tramadol similar to that with other opioids – Product monograph lists as warning/precaution Tramacet Precautions Serotonergic Syndrome – Patients may be at risk if Tramacet is coadministered with other serotonin increasing drugs • MAO inhibitors, SSRIs, meperidine – Spectrum of severity • • • • Mental changes: confusion, agitation Automonic effects: fever, sweating, labile vitals Motor effects: pyramidal rigidity, tremors Supportive treatment What about Codeine allergy? Is it safe to give Tramacet? Product Monograph states: “Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive Tramacet. Very cautious position, no evidence Morphine and it’s cousins much more likely to be of concern in severe codeine allergy. DO A HISTORY! 99% of patient reported codeine allergy are just S/E or MBE. CODEINE OXYCODONE MORPHINE TRAMADOL Tramadol Meperidine Fentanyl Tramacet Cost? Hospital gets a deal. Price matched with T # 3. Patient pays 62 cents per tab. Dispensing fee $15.00 + 60 tabs = $52.00 vs. about $18.00 for T#3. Discuss with patient? Acute Pain Treatment for the Ambulatory Patient Pre-op: 2 hours before – Celecoxib 400 mg or Ibuprofen 600 mg – Acetaminophen 975 mg or Tramacet 2 –3 Intra-op – Bupivacaine 0.5% epi, 0.5 ml/kg surgical wound infiltration, pre-incision better Post-op – Acetaminophen 650 – 975 mg Q6H – Ibuprofen 200 – 400 mg Q6H – Hydromorphone 1 or 2 mg tabs, 1 – 2 tabs Q3H OR – Ibuprofen or celecoxib/Tramacet/Hydromorphone The Tramacet Titration Tree Acetaminophen A 325 mg T T T A T A A A D T Tramacet D Dilaudid 2 mg T T T T A A A A The Tramacet Titration Tree Tramacet 2 tabs Q4H W/A – If patient reports pain 2/10 or less may replace one or two tabs with plain acetaminophen 325 mg per tab – If patient reports pain greater than 5/10 with activity, may add hydromorphone 1-2 mg PO Q4H prn Hyperalgesia Pro-nociceptive modulation Nociceptive Stimulus Anti-nociceptive modulation Pain Analgesia Analgesic Drugs that act by Nociceptive Modulation Pro-antinociceptive – Augments inhibitory modulation of nociception i.e opioids Anti-pronociceptive – Inhibits the facilitatory modulation of nociception i.e. ketamine, gabapentin and pregabalin Pregabalin for acute pain? Acute pain is “off-label” use Be cautious of Over-sedation – Sleep deprivation – Elderly – Patient already has significant opioids Pregabalin: The Good, The Bad and the Ugly The Good – happy patient – Chronic pain in region of surgery, when pronociceptive mechanisms play a role such as joint arthroplasty, bowel surgery in IBD patients, chronic limb ischemic pain, opioid tolerant patients The Bad – sedated patient – Mild pain when simple analgesics like acetaminophen, NSAIDs or low dose opioid or Tramacet suffice. The Ugly – ICU bound patient – Too large a dose in sleep deprived patient already in state of “morphine-failure” Pregabalin dosage This is NOT a one size fits all. – Drugs binding to receptors have considerable patient to patient variability in dose:response Alpha-2 delta sub-unit of Voltage-Gated Calcium Channel 75 mg PO 2 hours pre-op (50 – 150) 50 mg PO Q8H for 3 to 5 days (25 – 75) How do we stop all these drugs? 48 yr patient had a laparotomy for SBO and sent home on celecoxib, tramacet and hydromorphone How do we stop all these drugs? Last on first off and first on last off. – Discontinue hydromorphone first – Next reduce and stop Tramacet – NSAID – Acetaminophen Out-patient support – Family doctor? – APS nurse? Naloxone, a two-edged sword! Is there a down side to the administration of naloxone, 0.4 mg IV in the post-op patient where opioid induced respiratory depression is suspected? Severe acute pain, sympathetic response, pulmonary edema, MI, dysrhythmias Case Presentation: Somnolence and hypoxemia while on IV PCA Morphine 65 yr. Female with large ventral hernia repair on IV PCA morphine PMHx: Angioplasty 9 yr. ago, MI, CHF in past – Moderate COPD, NIDDM Doing well day 1, but day 2 found to be somewhat confused, somnolent and SaO2 remains in high 80s despite Oxygen by N/P Is Narcan Indicated? Urgently? Case Presentation: Somnolence and hypoxemia while on IV PCA Morphine Further patient evaluation – Patient arousable, RR 8-16, pupils slightly constricted, BP 130/70, pulse 90 and reg. – Chest: A/E fair bil. And some mild basilar creps – ABG: pH 7.46 pCO2 50 pO2 55 BiCarb 36 FiO2 > .50 – Chest X-ray: Extensive bilateral, diffuse, interstitial infiltrate consistent with ARDS Naloxone would probably have had a serious adverse effect on this patient. Hypoxemia despite supplemental O2 in a breathing patient. Look beyond the Opioids! Case Presentation: Somnolence and hypoxemia while on IV PCA Morphine Management of suspected opioid induced respiratory depression – – – – – Support A/W Stimulate breathing Supply supplemental oxygen Assess SaO2, BP, Pulse Naloxone titration, IF INDICATED • 0.04 mg Q5 min. X 3 as needed Hypoxemia is a medical emergency Hypercarbia is NOT ACUTE PAIN MANAGEMENT: SCIENTIFIC EVIDENCE 2nd Edition June ‘05 Australian and New Zealand College of Anaesthetists And Faculty of Pain Medicine. http://www.anzca.edu.au/publications/acutepain.pdf The above web site has the entire document and is freely Available to download. Useful texts Free!! From Canadian Pain Society Managing Pain: The Canadian Healthcare Professional’s Reference. Edited by Roman Jovey. 2008. Endorsed by the CPS. Order from Purdue Pharma Acute Pain Management: A practical guide. 3rd ed. 07 Pamela MacIntyre. Saunders/Elsevier Useful websites on Pain Prospect:Procedure Specific Post-op Pain Management http://www.postoppain.org/frameset.htm Pain Explained http://www.painexplained.ca/content.asp?node=4 The Canadian Pain Society http://www.canadianpainsociety.ca/indexenglish.h tml Useful websites on Pain Pain Institute http://www.medscape.com/infosite/paininstitute/article5?src=0_0_ad_ldr Internation Association for the Study of Pain http://www.iasppain.org//AM/Template.cfm?Section=Home