key concepts in acute pain management
advertisement

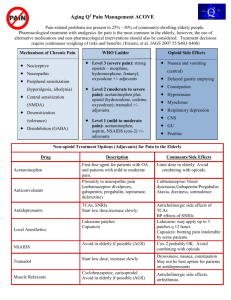
KEY CONCEPTS IN ACUTE PAIN MANAGEMENT - 1 SURGERY RESIDENTS Dec. 18, 2007 John Penning MD FRCPC Director Acute Pain Service Objectives General Key Concepts – The “real cost” of acute pain – Multi-modal analgesia Discuss key concepts of each modality – COX-inhibitor as foundational analgesic – Coxibs – “platelet sparing” cox-inhibitors – Tylenol # 3 has it’s limitations – Opioids – think outside the “box” – Tramacet – a “me too” drug? Or something to new to add? Consequences of poorly managed acute post-operative pain The Patient suffers – – – – – – CVS: MI, dysrhythmias Resp: atelectasis, pneumonia GI: ileus, anastamosis failure Endocrine: “stress hormones” Hypercoagulable state: DVT, PE Impaired immunological state • Infection, cancer, wound healing – Psychological: • Anxiety, Depression, Fatigue – Chronic Post-surgery/trauma Pain Consequences of poorly managed acute post-operative pain The Hospital – – – – – Increased costs $$$ Poor staff morale Reputation/Standing in the Community, Nationally Accreditation Litigation The Healthcare professional – Morale – Complaints to College – Litigation Benefits of Optimal Acute PostOperative Pain Management The Hospital – Increased patient satisfaction – Increased staff morale – Compliance with national guidelines, accreditation criteria – Cost Savings • Earlier ambulation and enteral feeding • Decreased complications/ICU expenditures • Decreased Length of Stay The New Challenges in Managing Acute Pain after Surgery and Trauma Patients/Society more “aware” of their rights to have good pain control – We are being held accountable Pressure from hospital to minimize length of stay – Control pain, limit S/E and complications The New Challenges in Managing Acute Pain after Surgery and Trauma The Opioid Tolerant Patient – The greatest change in practice/attitudes in the last 10 years is the now wide spread acceptance of the use of opioids for CHRONIC NON-MALIGNANT PAIN – Renders the “usual” standard “box” orders totally inadequate in these patients Get an accurate Drug History – The Brief Pain Inventory – “BPI” What is the “Best Way” to manage acute post-operative pain? FIRST, DO NO HARM Therefore, the “best way” is a BALANCE Patient Safety Effective Analgesic Modalities KEY POINTS “Emphasis is placed on the utilization of a multimodal analgesic approach to maximize analgesia while minimizing side-effects.” – Transduction – Transmission – Modulation – Perception There is as of yet no single silver bullet!! Pain Pathways Acute Pain Management Modalities Cyclo-oxygenase inhibitors – Non-specific COX inhibitors(classical NSAIDs) – Selective COX-2 inhibitors, the “coxibs” – Acetaminophen is probably COX-3 Local anesthetics Opioids NMDA antagonists – Ketamine, dextromethorphan Anti-convulsants – Gabapentin, Pregabalin Tissue Trauma Cell Membrane Phospholipids Phospholipase Arachidonic Acid C O X Cyclo-oxygenase Endoperoxides Thromboxane Prostaglandins Toxic Oxygen Radicals Prostacyclin Analgesia with Opioids alone The harder we “push” with single mode analgesia, the greater the degree of side-effects Side-effects Analgesia Multi-modal Analgesia “With the multimodal analgesic approach there is additive or even synergistic analgesia, while the sideeffects profiles are different and of small degree.” Side-effects Analgesia Case Problem: Severe Respiratory Depression after Toradol? Healthy 34 yr. patient c/o severe incisional pain in PACU after ovarian cystecomy Received 200 g fentanyl with induction and 10 mg morphine during case PCA morphine started in PACU, plus nurse supplements totaled 26 mg in 90 minutes Still c/o pain, 30 mg Toradol IM given with some relief after 15 minutes, so patient sent to ward 60 minutes later found unresponsive, cyanotic, RR 4/min. Case Problem: Severe Respiratory Depression after Toradol? Pharmacodynamic drug interaction between morphine and NSAID – morphine’s respiratory depressant effect opposed by the stimulatory effects of pain, busy PACU environment – NSAID decreases pain, morphine’s effect unappossed Gain control of acute pain with fast onset, short acting opioid(fentanyl) Add NSAID adjunct early Monitor closely for sedation and respiratory depression after pain is alleviated by any means The problem with the “Little Pain – Little Gun”, “Big Pain – Big Gun” Approach With opioids analgesic efficacy is limited by side-effects “Optimal” analgesia is often difficult to titrate – 10 – fold variability in opioid dose:response for analgesia – A dose of opioid that is inadequate for patient A can lead to significant S/E or even death in patient B. • Many patient factors add to the difficulty – Opioid tolerance, anxiety, obstructive sleep apnea, sleep deprivation, concomitantly administered sedative drugs The rationale for COX-Inhibitors in acute pain management The problem with the “Little Pain – Little Big Pain – Big Gun Approach” Gun, – Patient Safety!! If the “Big Gun” is failing due to dose limiting sedation/respiratory depression, the addition at that time of the “Little Gun” may kill the patient. NSAID and Acetaminophen CONCEPT # 1 The foundation of all acute pain Rx protocols. ”First on last off” sole agent in mild /moderate pain Analgesic efficacy is limited inherently In contrast, with opioids efficacy is limited by S/E Opioids added as required opioid sparing effect 30-60 % # of Deaths in thousands Mortality From NSAID-Induced GI Complications vs Other Diseases in US 25 20 15 10 5 0 Leukemia HIV NSAIDs- Multiple Asthma Myeloma GI Cause of Deaths Wolfe MM: NEJM 1999; 340: 1888-99 Cervial Cancer Penning’s Pessimistic Policy on Pain Pills Pick your “Poison” Pursuant to Patient Profile COX-inhibitors are potential killers “in the long run” Opioids are potential killers “in the short run” Cyclo-oxygenase inhibitors Acetaminophen Naproxen Celecoxib Ketorolac Rofecoxib Cell Membrane Phospholipids Phospholipase Arachidonic Acid COX-1 COX-2 Prostaglandins Prostaglandins Gastric Protection Platelet Hemostasis Acute Pain Inflammation Fever Why a COX-2 inhibitor? Equivalent analgesic efficacy with nonselective COX-inhibitors No effects on platelets! Better GI tolerability – Less dyspepsia, less N/V Two hours before surgery associated with post-op pain 1. Celecoxib 400 mg PO If severe allergy to sulfa? 2. Naproxen 500 mg PO Contra-indications to NSAID Acetaminophen 1000 mg PO Drug Summary Relative Risk for Cardiovascular Event (95% CI) Rofecoxib, ≤ 25 mg 1.33 (1.00 - 1.79) Rofecoxib, > 25 mg Celecoxib Diclofenac 2.19 (1.64 - 2.91) Naproxen Piroxicam Ibuprofen 0.97 (0.87 - 1.07) 1.06 (0.70 - 1.59) 1.07 (0.97 - 1.18) Meloxicam 1.25 (1.00 - 1.55) Indomethacin 1.30 (1.07 - 1.60) 1.06 (0.91 - 1.23) 1.40 (1.16 - 1.70) *CI indicates confidence interval. Source: JAMA. Published online September 12, 2006 (McGettigan and Henry). Contra-indications to Celecoxib/NSAIDs Patients with the “ASA triad” – Risk of severe asthma, angioedema precipitated with COX-inhibitor Renal insufficiency or risk there of – especially if risk of hypovolemia periop – Vascular patients having aortic cross-clamp and/or probable angiogram peri-operatively Poorly controlled hypertension – Especially if pt. is on ACE inhibitor, potent loop diuretics Contra-indications to Celecoxib/NSAIDs Congestive heart failure Active peptic ulcer disease Risk of non-union in bone surgery or non-fusion in spine surgery – COX-1 proven a problem in high doses – COX-2? Proven OK for 5 days Celecoxib and “sulfa allergy” Allergy to sulfa?? History, Please! – Most allergies are bogus: N/V, diarrhea – A rash with sulfonamide anti-biotics? Celecoxib belongs to the “other” class of sulfonamides: furosemide, glyberide, etc. – Do not use celecoxib is history of anaphylaxis or severe cutaneous reaction (Steven-Johnson sydrome. etc.) with a sulfonamide The Opioids We have to stop trying to put every patient in the “analgesic dose box” Meperidine 75 mg IM Q4H prn Tylenol #3 1 – 2 PO Q4H prn Opioids CONCEPT # 2 Pharmacokinetic + Pharmacodynamic patient to patient variability results in 1000 % variability in opioid dose requirements (standardized procedure, opioid naïve patient) – opioid dosage must be individualized – therefore, if parenteral therapy indicated, IV PCA much better suited to individual patient needs than IM/SC Opioids *Cancer Pain Monograph (H&W, 1984) CONCEPT # 3 Under utilization of high efficacy PO opioids PO opioid equivalence of 10 mg morphine IM/SC * Morphine 20 mg Hydromorphone 4 mg oxycodone 10 mg meperidine 200 mg codeine 200 mg True or False? One opioid is just like any other, in terms of analgesic efficacy and side-effects. Opioids – Are they all the same? Morphine Hydromorphone (dilaudid) Fentanyl Oxycodone (parenteral n/a) Meperidine (demerol) Opioids – Do they all act the same? Opioids work as analgesics by activating endogenous inhibitory pain modulating systems Opioid receptors – Mu, Delta and Kappa – Large genetic variability in expression Good choice in one patient may be poor choice in another – Analgesic efficacy – Side-effect profile Meperidine Morphine Atropine Fentanyl Bupivacaine True or False? One opioid is just like any other, in terms of analgesic efficacy and side-effects. Answer. There is considerable variability between patients in response to different opioids. True or False? Meperidine should be eliminated from the hospital formulary? Meperidine Pharmacology Opioid agonist – Mu and some kappa NMDA antagonist (weak) Local anesthetic action – equipotent to lidocaine SSRI (weak) Muscaric blockade – “atropine-like” – Central anti-cholinergic effects often causes confusion in the elderly Meperidine’s major problem Normeperidine – The “ugly” metabolite • Neuroexcitatory: twitches, dilated pupils, hallucinations, hyperactive DTR, seizures • Non-opioid receptor mediated, no tolerance • Half-life is 15 – 20 hours N-demethylation Meperidine and MAO Inhibitors Meperidine blocks the neuronal re-uptake of serotonin, may result in serotonergic crisis in patients being treated with MAO inhibitors – Excitatory reaction with delirium, hyper or hypo tension, hyperthermia, rigidity, seizures, coma, death – Supportive management, ? Benzos, dopaminergics? When to use Meperidine? As a third line opioid when other choices have failed – Especially if patient has Hx of such Less than 600 mg per day Short duration of 2 days or less Avoid in elderly or renal failure patients May be useful in small IV doses to supplement other opioids – 25 mg IV Q1H prn Who still uses Tylenol # 3 ? WHY ?? Opioid Myths that still prevail! Codeine is a “weak” opioid? Codeine is inherently safer than the more potent opioids? CODEINE – A drug whose time has come and gone? N Engl J Med 351; 27 Dec. 30, 2004 Problems with Codeine 62 yr. male with CLL, presents with bilateral pneumonia. Broncho-lavage revealed yeast – Anti-biotics: Ceftriaxone, clarithromycin, voriconazole – Codeine 25 mg PO TID for cough Problems with Codeine Day 4 became markedly sedated, pinpoint pupils and ABG reveals PaCO2 of 80 mmHg. Marked improvement with Naloxone. What’s the expected morphine blood level? Answer: 1 to 4 mcg/L This patient’s morphine blood level? – 80 mcg/L Codeine Metabolism in Normal Circumstances The major pathways convert codeine to inactive metabolites – CYP3A4 pathway yields norcodeine – Glucuronidation The minor pathway, about 10%, yields morphine – CYP2D6, essential for analgesic effect 60 mg Codeine PO – approx. 4 mg morphine SC Variability! 60 mg PO Codeine yields potentially 0 to 60 mg parenteral morphine Genetic Variability And drug interactions 1% Finland 10% Greek 30% East Africa Potential Codeine Drug Interactions Major pathway – CYP3A4 – Inducers decrease codeine effect – Inhibitors increase codeine effect Minor pathway - CYP2D6 – Inducers increase codeine effect – Inhibitors decrease codeine effect Inhibitors of CYP2D6 SSRIs (potent) especially PAXIL Cimetidine, Ranitidine Desipramine Propranolol Quinidine (potent) Viagra Many anti-biotics and chemo Why not just go with Percocet? Too potent for some patients – 5 mg oxycodone = 60 mg codeine It too, may be a pro-drug? – Codeine is to Morphine as – Oxycodone is to ?? Oxymorphone – The jury is still out on this one Instead of Tylenol # 3 ? Acetaminophen 650 mg PO Q4H with Morphine 10 – 20 mg PO Q4H prn OR Dilaudid 2 – 4 mg PO Q4H prn Newly available Tramacet 1 – 2 tabs PO Q4H prn Opioids STOP Hydromorphine 1 – 4 mg PO/IM/IV Q4H prn NOT! This represents up to 30 fold range in peak effect in any given patient 1 mg PO ---- 4 mg IV bolus homeopathic dose ---- potentially lethal Opioids: Rational multi-route orders? Foundation of Acetaminophen/NSAID Morphine 5 - 10 mg PO Q4h prn Morphine 2.5 - 5 mg s.c. Q4h prn Morphine 1-2 mg IV bolus Q1h prn Hydromorphone 1 - 2 mg PO Q4h prn Hydromorphone 0.5 – 1 mg s.c Q4h prn Hydromorphone 0.25 – 0.5 mg IV Q1h prn Towards a better analgesic for acute pain High level of efficacy A good drug would have an inherent multi-modal mechanism of action Very low risk of serious side-effects Low incidence of bothersome sideeffects Very limited abuse potential Affordability TRAMADOL What about Tramacet? Combination drug, 325 mg of acetaminophen + 37.5 mg of tramadol Ordered like T#3 – 1 to 2 tabs Q4H prn Efficacy limited by max dose for acetaminophen. Opioids can be added as required! Is Tramadol New? Just recently available in Canada, as Tramacet Synthesized in 1962, available in Germany since 1977, UK 94, US 95 where IV formulation is also available Minimal risk of respiratory depression and abuse potential, never been a “scheduled” drug Now #1 prescribed centrally acting analgesic worldwide > 50 million patients Tramacet - How does it work? Inherent multimodal action – 4 distinct mechanisms 1. acetaminophen 2. Weak mu agonist – very weak opioid 3. Augments endogenous inhibitory nociceptive modulation via serotonin 4. and norepinephrine pathways Advantages of Tramacet? Tramadol’s “strength” lies in it’s “weakness” as an opioid – Poor Mu receptor affinity Minimal opioid effect – Less constipation, faster return to normal bowel function – Less N/V – No sig. respiratory depression – No sig. risk for abuse (not classified as narcotic) Advantages of Tramacet? Tramadol’s “strength” lies in it’s “weakness” as an opioid – Poor Mu receptor affinity Tramadol does not antagonize the action of classic mu agonists like morphine, dilaudid or fentanyl – Unlike the partial agonist/antagonists such as Talwin, Nubain, Stadol Other mu agonist may be added Does Tramacet work? Combination tramadol plus acetaminophen for postsurgical pain. Adam B. Smith et al. The American Journal of Surgery 2004; V187: 521 – 527. 1 tab of Tramacet = 1 tab T #3 – IN YOUR AVERAGE PATIENT !! Tramacet Precautions Liver Toxicity – Risk of acetaminophen dose exceeding recommended 4 gm/day in 70 kg patient, if patient inadvertently takes other acetaminophen products, especially OTC. Risk of seizures, very rare – U.K. Safety Committee reports 1:7000 – Most cases involving interaction with proconvulsant agents or large IV doses of tramadol – Risk taking tramadol similar to that with other opioids – Product monograph lists as warning/precaution Why combination analgesics are not a great idea Acetaminophen-Induced Acute Liver Failure: Results of a USA Multicenter, Prospective Study. Hepatology, Vol. 42, No. 6, 2005. Larson et al. 22 centers, 662 cases ’98 – ’03. 50% cases due to acetaminophen 50% of acetaminophen cases inadvertent Tramacet Precautions Serotonergic Syndrome – Patients may be at risk if Tramacet is coadministered with other serotonin increasing drugs • MAO inhibitors, SSRIs, meperidine – Spectrum of severity • • • • Mental changes: confusion, agitation Automonic effects: fever, sweating, labile vitals Motor effects: pyramidal rigidity, tremors Supportive treatment What about Codeine allergy? Is it safe to give Tramacet? Product Monograph states: “Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive Tramacet. Very cautious position, no evidence Morphine and it’s cousins much more likely to be of concern in severe codeine allergy. DO A HISTORY! 99% of patient reported codeine allergy are just S/E or MBE. CODEINE OXYCODONE MORPHINE TRAMADOL Tramadol Meperidine Fentanyl Tramacet Cost? Hospital gets a deal. Price matched with T # 3. Patient pays 62 cents per tab. Dispensing fee $15.00 + 60 tabs = $52.00 vs. about $18.00 for T#3. Discuss with patient? Acute Pain Treatment for the Ambulatory Patient Pre-op: 2 hours before – Celecoxib 400 mg or Ibuprofen 600 mg – Acetaminophen 975 mg or Tramacet 2 –3 Intra-op – Bupivacaine 0.5% epi, 0.5 ml/kg surgical wound infiltration, pre-incision better Post-op – Acetaminophen 650 – 975 mg Q6H – Ibuprofen 200 – 400 mg Q6H – Hydromorphone 1 or 2 mg tabs, 1 – 2 tabs Q3H OR – Ibuprofen or celecoxib/Tramacet/Hydromorphone The Tramacet Titration Tree Acetaminophen A 325 mg T T T A T A A A D T Tramacet D Dilaudid 2 mg T T T T A A A A ACUTE PAIN MANAGEMENT: SCIENTIFIC EVIDENCE 2nd Edition June ‘05 Australian and New Zealand College of Anaesthetists And Faculty of Pain Medicine. http://www.anzca.edu.au/publications/acutepain.pdf The above web site has the entire document and is freely Available to download. Opioid Conversions – Parenteral to Oral and Equivalents (approx.) Morphine 10 mg Morphine 20 mg Hydromorphone 2 mg Hydro…. 4 mg Meperidine 75 mg Meperidine 200 mg Codeine 120 mg Codeine 200 mg Oxycodone (n/a) Oxycodone 10 mg Opioid Conversions – Oral to Parenteral and Equivalents (approx.) Morphine 40 mg Hydromorphone 8 mg Meperidine 300 mg Codeine 300 mg Oxycodone 15 mg Morphine 10 mg Hydro…. 2 mg Meperid.. 75 mg Codeine 120 mg Oxycodone (n/a)