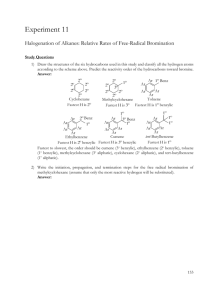
Hydrocarbon Unsaturation Test: Bromine Experiment
advertisement

Experiment - Testing Hydrocarbons for Unsaturation using Bromine Topic: 10.2 Organic Chemistry Time Allowed: 15 minutes Description: A solution of bromine in hexane (“bromine water”) is used to detect whether an organic compound is unsaturated. Unsaturated compounds rapidly decolourise the bromine. Apparatus (per group): One plastic or ceramic well plate. One empty plastic pipette Chemicals (per group): Solutions and liquids contained in plastic pipettes: Cyclohexane Cyclohexene Limonene Solution of bromine in hexane (“bromine water”). Safety: Wear lab coats and eye protection. It is the responsibility of the teacher to carry out a risk assessment. Procedure: 1. Using a plastic pipette, add three drops of the bromine solution to each of three wells in a well-plate. 2. Put three drops of each of the organic liquids under test into the wells and observe any changes. Discussion Question: 1. Which types of liquid decolourise bromide?