03h
advertisement
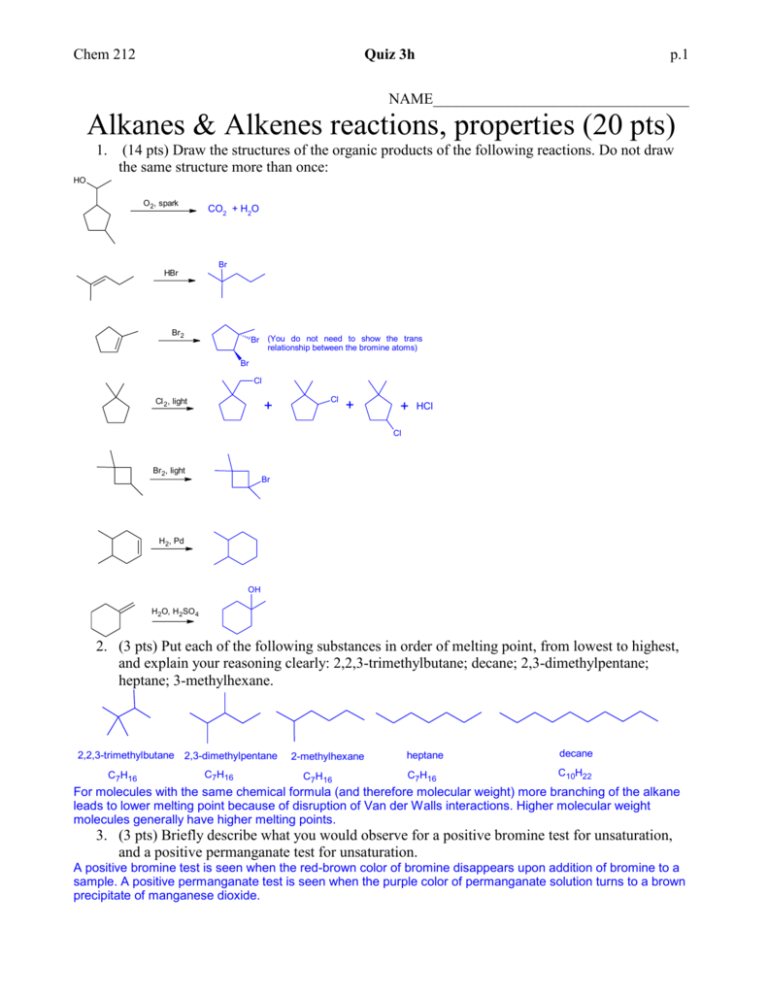
Chem 212 Quiz 3h p.1 NAME__________________________________ Alkanes & Alkenes reactions, properties (20 pts) 1. (14 pts) Draw the structures of the organic products of the following reactions. Do not draw the same structure more than once: HO O 2, spark CO2 + H2O Br HBr Br2 (You do not need to show the trans relationship between the bromine atoms) Br Br Cl Cl 2, light + Cl + + HCl Cl Br2, light Br H2, Pd OH H2O, H 2SO 4 2. (3 pts) Put each of the following substances in order of melting point, from lowest to highest, and explain your reasoning clearly: 2,2,3-trimethylbutane; decane; 2,3-dimethylpentane; heptane; 3-methylhexane. 2,2,3-trimethylbutane 2,3-dimethylpentane C7H16 C7H16 2-methylhexane C7H16 heptane decane C7H16 C10H22 For molecules with the same chemical formula (and therefore molecular weight) more branching of the alkane leads to lower melting point because of disruption of Van der Walls interactions. Higher molecular weight molecules generally have higher melting points. 3. (3 pts) Briefly describe what you would observe for a positive bromine test for unsaturation, and a positive permanganate test for unsaturation. A positive bromine test is seen when the red-brown color of bromine disappears upon addition of bromine to a sample. A positive permanganate test is seen when the purple color of permanganate solution turns to a brown precipitate of manganese dioxide.











