Alkenes Worksheet: Reactions, Identification, and Polymers
advertisement

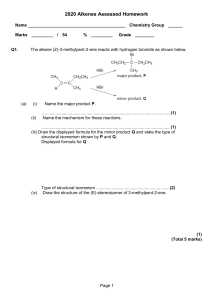
Questions on Alkenes 1. State the equation and name the organic product when propene reacts with: (a) hydrogen using a nickel catalyst at 180 oC. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ (b) chlorine gas in the absence of water. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2. Describe how you could distinguish practically between hexane and hex-3-ene by using a simple chemical reaction. Describe what you would observe in each case and state any relevant equations and name any products formed. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 3. Ethanol is increasingly being produced commercially by the fermentation of crops in order to use as a biofuel in place of/in addition to gasoline (petrol). This is ecologically friendly in terms of greenhouse gas emissions but it is using up vast areas of land which could be used for food production. Ethanol can also be made industrially by the hydration of ethene. State the equation for the hydration of ethene. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 4. (a) State the equation and name the organic product when but-2-ene reacts with hydrogen iodide. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ (b) Unlike the reaction with but-2-ene, the reaction of but-1-ene with hydrogen iodide is not on the IB Core. This is because in theory it can give two different organic products although in practice only one of them is formed. Deduce the identity of the two possible products. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 5. (a) Draw the structure of the repeating unit of poly(chlorethene). _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ (b) Deduce the structure of the repeating unit of poly(tetrafluoroethene). _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ (c) Poly(tetrafluoroethene) is also known as ‘Teflon’ or ‘non-stick’ and it is used to make artificial joints for humans as well as coat frying pans. Suggest a reason why it is so unreactive. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Answers 1. (a) CH3CH=CH2(g) + H2(g) → CH3CH2CH3(g) Product: propane (b) CH3CH=CH2(g) + Cl2(g) → CH3CHClCH2Cl(l) Product: 1,2-dichloropropane 2. Add a small amount of bromine water to each substance. The bromine will dissolve in the hexane to form a yellow-brown solution but no reaction takes place. The hex-3-ene solution will decolourise the bromine water. This is due to the addition of the bromine water to the double bond of the hex-3-ene to form 4-bromohexan-3-ol. CH3CH2CH=CHCH2CH3(l) + Br2(aq) + H2O(l) → CH3CH2CHBrCH(OH)CH2CH3(l) + HBr(aq) (The IB would also accept 3,4-dibromohexane, CH3CH2CHBrCHBrCH2CH3, as the product as this would be formed if no water is present). 3. H2C=CH2(g) + H2O(g) → C2H5OH(l) 4. (a) CH3CH=CHCH3(g) + HI(g) → CH3CHICH2CH3(l) Product: 2-iodobutane (b) 1-iodobutane, CH2ICH2CH2CH3 and 2-iodobutane, CH3CHICH2CH3 5. (a) ―(―CH2―CHCl―)n― (b) ―(―CF2―CF2―)n― (c) The very strong C-F bond is difficult to break or the structure is such that there is no room around each carbon atom for other atoms to approach.