Chemistry 1212 – Principles of Chemistry II Section B, Summer 2014
advertisement

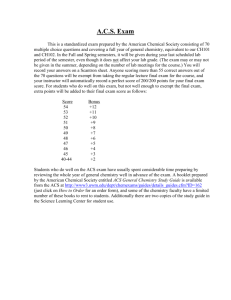
Chemistry 1212 – Principles of Chemistry II Section B, Summer 2014 11:05 am – 1:15 pm MTWR (Rogers 307) Lecturer: Office Hours: Office: Email: Dr. Royce Dansby-Sparks By Appointment Rogers 309, Phone # 706-864-1492 Royce.Dansby-Sparks@ung.edu Course Description: Second course in a two-semester sequence covering the fundamental principles and applications of chemistry for science majors. Topics to be covered include states of matter, properties of solutions, kinetics, chemical equilibrium, acids/bases/buffers, titrations, solubility, thermodynamics, and electrochemistry. Course Objectives: Upon successful completion of this course, students should understand: • • • • • • • The properties and behavior of liquids and solids; Properties and behavior of solutions and solubility; The fundamentals of chemical kinetics and rate laws; The nature of reactions at equilibrium; The characteristic properties of acids and bases; Thermodynamics and the interconversion of heat and energy; The basics of electrochemistry – the transfer of electrons. Corequisite: Principles of Chemistry II Laboratory (CHEM 1212L). All students are required to be simultaneously enrolled in BOTH 1212 and 1212L. Materials: 1. 2. 3. 4. th Chemistry, 6 Ed., McMurry & Fay, Prentice Hall, 2011. Subscription to Mastering Chemistry (included if text is purchased from the bookstore) Simple scientific calculator (see below) Access to UNG D2L Course Coverage: Ch 10 – Liquids, Solids, and Phase Changes Ch 11 – Solutions and their Properties Ch 12 – Chemical Kinetics Ch 13 – Chemical Equilibrium Ch 14 – Aqueous Equilibria: Acids and Bases Ch 15 – Applications of Aqueous Equilibria Ch 16 – Thermodynamics: Entropy, Free, Energy, and Equilibrium Ch 17 - Electrochemistry Methods of Instruction: The laboratory portion of this course is graded separately from the lecture portion. Your understanding of the materials presented in the book and lecture will be evaluated by your performance on quizzes, homework, tests, and a comprehensive final exam. Grading: In-class and on-line quizzes (approximately every week) D2L and Mastering Chemistry Assignments Hour Exams (4) Final Exam Percent total grade 15 % 10 % 60 % 15 % -Letter grades for the course will be assigned according to the following absolute scale: >90.0% - A 80.0-89.9% - B 67.0-79.9% - C 57.0-66.9% - D <57% - F -Grade cut-offs will be evaluated at the end of the semester and might be lowered slightly (typically less than 1 point if at all) based on the grade distribution of the class. Quizzes: In-class and on-line quizzes will be administered periodically. Quizzes may or may not be announced. There are no make-ups for missed quizzes with the exception of previously arranged absences for University sanctioned events. The lowest quiz grade will be dropped, including any zeros for absences. Hour Exams: Four in-class exams will be given. A pen/pencil and calculator will be allowed for these exams. Each exam will be exactly 75 minutes long and will be given during the lecture time period. Exact coverage is listed below and will be confirmed several days before the exam. NOTE: Make-up exams will only be given for absences noticed/approved in advance and with instructor approval of documentation within two days of the exam. These exams may be different than the exams given in class. This means you MUST provide a doctors office documented and acceptable excuse, even if you would typically self-treat your illness. Final (ACS standardized) Exam: The American Chemical Society (ACS) standardized exam will be given during the final exam period. The ACS exam will be cumulative multiple-choice and covers all topics from both semesters of general chemistry. Please take this exam very seriously! Study guides are available for purchase from the Society of Chemistry Students (SCS) Club. Academic Integrity Policy: Honor Code: “On my honor, I will not lie, cheat, steal, plagiarize, evade the truth or tolerate those who do.” Suspected violations of the Academic Integrity policy should be referred by students to the instructor. If the instructor concludes that a violation of the Academic Integrity policy has occurred, the instructor will either (1) penalize the student and file an incident report with the Academic Integrity Council and/or (2) refer the matter directly to the Academic Integrity Council. If the instructor files an incident report, the instructor will review the completed report with the student and will request that the student sign the report as an indication that the student is aware of the contents of the report. Any violation or attempted violation will result in an F for the course. Attendance Policy: Regular attendance will be essential to obtaining a satisfactory grade in this course. Official University policy states that attendance is mandatory. Attendance will only be tracked during the University’s role verification process. After this time, it is the responsibility of the student to ensure success in the course by regularly attending lectures. Students will be responsible for obtaining any missed course content from their peers and zeros will be given for any in class assignments that are missed during an absence, except as noted above. In accordance with University policy, a grade of W/WF can be given if a student misses 10% or more of the course. Calculator Policy: In chemistry courses numbered 1212 (CHEM 1212) or lower, only non-programmable, 1- or 2line scientific calculators may be used for in-class assignments, including all quizzes and exams. A scientific calculator is one that can calculate the values of standard algebraic and log and exponent functions, but cannot display graphs or functions or do symbolic manipulation. Cell Phones: All cell phones should be set to vibrate during class. Adult-like judgment and behavior will be rewarded with adult-like treatment in this area. Any student participating in disruptive activities will be asked to leave the class immediately. Accommodations for Students with Disabilities: This university is committed to equal access to its programs, services, and activities, and welcomes otherwise qualified students with disabilities. Students who require accommodations and services must register with Disability Services and submit supporting documentation. Disability Services provides accommodation memos for eligible students to give to their instructors. Students are responsible for making arrangements with instructors, and must give reasonable prior notice of the need for accommodation. Contact Information for Disability Services: Dahlonega Campus: Thomas McCoy, Assistant Director, tmmccoy@northgeorgia.edu Stewart Student Success Center, Room 313, 706-8672782. Supplemental Syllabus Information: Students are expected to refer to UNGs online supplemental syllabus for additional information: http://ung.edu/academic-affairs/policies-and-guidelines/supplemental-syllabus.php Class Evaluations: Class evaluations at North Georgia are now conducted online through Banner. Evaluation of the class is considered a component of the course and students will not be permitted to access their course grade until the evaluation has been completed. The evaluations will be accessible beginning one week prior to Final Exam week Chemistry 1212, Summer 2015 Lecturer: Dr. Royce Dansby-Sparks Tentative Lecture Schedule – See also complete schedule Date: T – May 26 W – May 27 R – May 28 M – Jun 1 T – Jun 2 W – Jun 3 R – Jun 4 M – Jun 8 T – Jun 9 W – Jun 10 R – Jun 11 M – Jun 15 T – Jun 16 W – Jun 17 R – Jun 18 M – Jun 22 Lecture Coverage Introduction & Chapter 10 Chapters 10 & 11 Chapter 11 & Review Exam1 & Chapter 12 Chapter 12 Chapter 13 Chapter 13 & Review Exam 2 & Chapter 14 Chapter 14 Chapter 15 Chapter 15 Review for Exam 3 Exam 3 & Chapter 16 Chapter 16 & 17 Chapter 17 Exam 4 & Review for Final Notes drop/add ends 5pm Exam 1 (10-11) Exam 2 (12-13) Last day to drop with “W” Exam 3 (14-15) Exam 4 (16-17) Tuesday June 23, 11:05-1:15 pm – ACS Standardized Final Exam Homework Problems: On-line homework will be assigned through Mastering Chemistry. I will also make suggested extra credit homework problems available for each chapter. If the EC problem sets from all chapters are completed before each of the four exams, I will assign 1-5 % extra credit points to your exam score based on your score on the selected problems. -Mastering Chemistry Course Code: MCDANSBYSPARKS38981