Oxidative Cleavage of Alkenes/Alkynes: Ozonolysis & KMnO4
advertisement
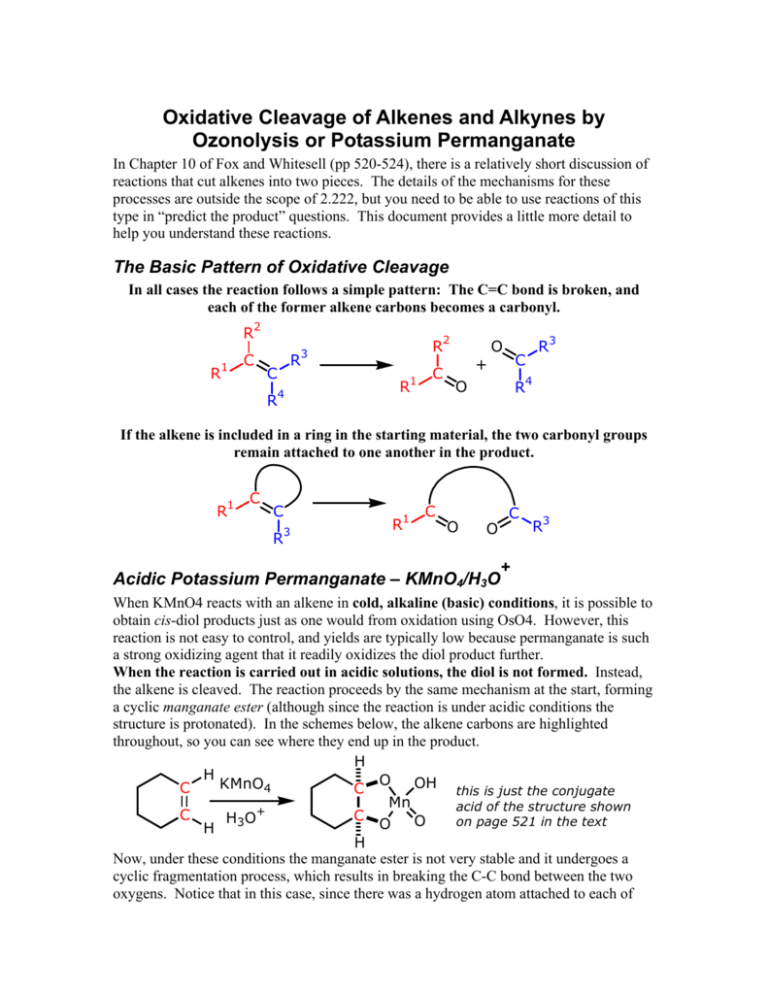
Oxidative Cleavage of Alkenes and Alkynes by Ozonolysis or Potassium Permanganate In Chapter 10 of Fox and Whitesell (pp 520-524), there is a relatively short discussion of reactions that cut alkenes into two pieces. The details of the mechanisms for these processes are outside the scope of 2.222, but you need to be able to use reactions of this type in “predict the product” questions. This document provides a little more detail to help you understand these reactions. The Basic Pattern of Oxidative Cleavage In all cases the reaction follows a simple pattern: The C=C bond is broken, and each of the former alkene carbons becomes a carbonyl. R R1 2 C 2 R C R 4 R 3 R1 C + O R3 C R4 O If the alkene is included in a ring in the starting material, the two carbonyl groups remain attached to one another in the product. R1 C C R 3 R1 C O C O 3 R + Acidic Potassium Permanganate – KMnO4/H3O When KMnO4 reacts with an alkene in cold, alkaline (basic) conditions, it is possible to obtain cis-diol products just as one would from oxidation using OsO4. However, this reaction is not easy to control, and yields are typically low because permanganate is such a strong oxidizing agent that it readily oxidizes the diol product further. When the reaction is carried out in acidic solutions, the diol is not formed. Instead, the alkene is cleaved. The reaction proceeds by the same mechanism at the start, forming a cyclic manganate ester (although since the reaction is under acidic conditions the structure is protonated). In the schemes below, the alkene carbons are highlighted throughout, so you can see where they end up in the product. H H O OH this is just the conjugate KMnO4 C C Mn acid of the structure shown + C C H3 O on page 521 in the text O O H H Now, under these conditions the manganate ester is not very stable and it undergoes a cyclic fragmentation process, which results in breaking the C-C bond between the two oxygens. Notice that in this case, since there was a hydrogen atom attached to each of the alkene carbons in the starting material, there is a hydrogen attached to the carbonyl carbon in the product and therefore the product is an aldehyde. O H C OH O OH C H H + Mn Mn C C O O O O H Aldehydes are very easily oxidized to carboxylic acids, and thus the aldehydes formed in the cleavage reaction do not survive. They are rapidly transformed into carboxylic acid groups, by a complex reaction whose mechanism you need not worry about. O O C C H KMnO4 H C C O OH OH O Now, if the alkene had not had any hydrogens attached, the product in that case would have been a ketone rather than an aldehyde. Ketones are not easily oxidized further, and the reaction would have stopped at that stage. CH3 O C CH3 KMnO4 CH3 O OH OH H3O+ C C CH3 + Mn Mn C C C O O CH3 O O CH3 If one of the alkene carbons had a hydrogen substituent, while the other did not, then we would get both acid and ketone groups in our product, as shown below. C C CH3 H KMnO4 H3O + CH3 O OH C Mn C O O H O C CH3 C CH3 OH H C O O C Ozonolysis Ozone (O3) is a much better behaved reagent than permanganate, at least at low temperatures. The fundamental pattern is identical to that of the permanganate reaction, although the details of the reaction mechanism are quite different. Ozone also cleaves alkenes, but it will not oxidize aldehyde groups to carboxylic acids. The reaction of O ozone with an alkene does not directly form carbonyl groups, however. It is necessary to reduce an intermediate ozonide, so this is always a 2-stage process. The reduction step simply cleaves the relatively weak peroxidic O-O bond in the ozonide. O CH3 C CH3 CH3 O3 Zn CH3 O CH Cl 2 2 C C HOAc C O H O O C C -78 oC reduction C O O H C O H H "molozonide" "ozonide" Don’t worry about exactly how the molozonide rearranges to form the ozonide. Simply notice that the alkene carbons of the starting material still appear as the carbonyl carbons in the product. Oxidative Cleavage Reactions of Alkynes An alkyne is more highly oxidized than an alkene, so it should be no surprise that when it is oxidatively cleaved, the products obtained are also at a higher oxidation level. Ozonolysis/reduction cleaves alkynes, forming carboxylic acid groups. 1) O3, CH2Cl2 R 1 C C 2 R O o -78 C 2) Zn, HOAc R1 O OH + HO C C R2 The intermediate structures are similar to those formed in the ozonolysis of alkenes, but simply involve addition of a second molecule of ozone. Notice that the net result is to form 3 C-O bonds at each of the former alkyne carbons, and the result of reduction with Zn/HOAc is to cleave the peroxidic bonds, just as before. You are not expected to be able to write detailed stepwise mechanisms for the rearrangement or reduction steps in this reaction. 1 R C C R2 O3 O R 1 C O O C R2 O3 O 1 R O C O C O O O R 2 O ozonide intermediates R 1 O C O O C O O 2 R








