Reading Chemical Formulas
advertisement
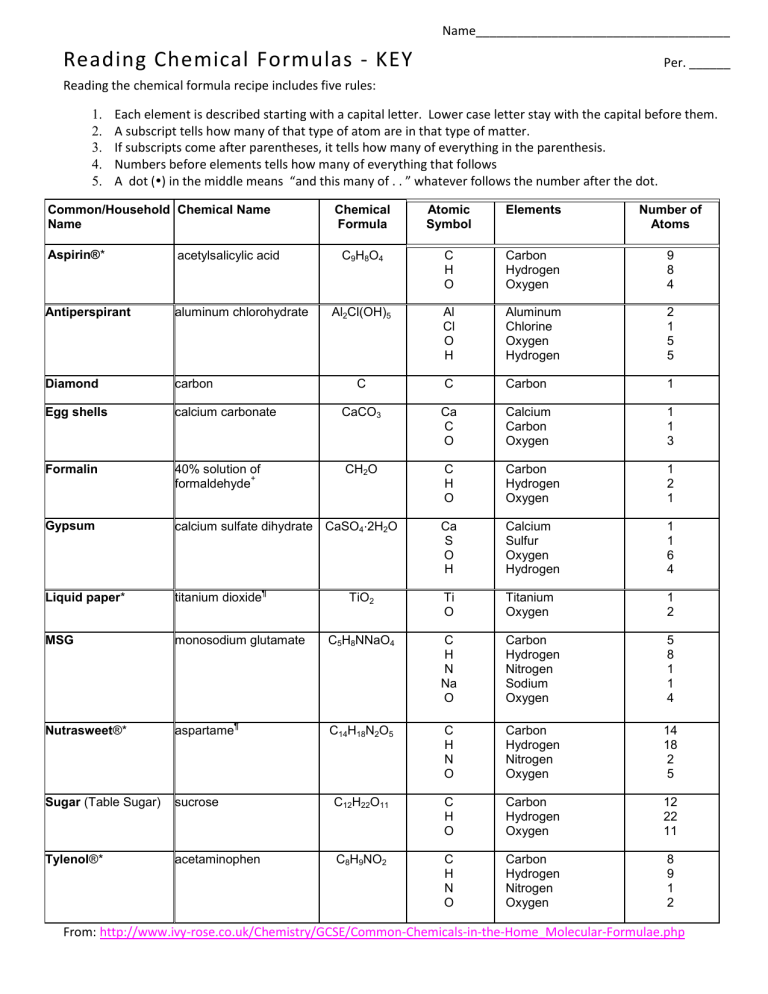
Name_____________________________________ Reading Chemical Formulas - KEY Per. ______ Reading the chemical formula recipe includes five rules: 1. 2. 3. 4. 5. Each element is described starting with a capital letter. Lower case letter stay with the capital before them. A subscript tells how many of that type of atom are in that type of matter. If subscripts come after parentheses, it tells how many of everything in the parenthesis. Numbers before elements tells how many of everything that follows A dot () in the middle means “and this many of . . ” whatever follows the number after the dot. Common/Household Chemical Name Name Chemical Formula Atomic Symbol Elements Number of Atoms C9H8O4 C H O Carbon Hydrogen Oxygen 9 8 4 Al2Cl(OH)5 Al Cl O H Aluminum Chlorine Oxygen Hydrogen 2 1 5 5 C C Carbon 1 Aspirin®* acetylsalicylic acid Antiperspirant aluminum chlorohydrate Diamond carbon Egg shells calcium carbonate CaCO3 Ca C O Calcium Carbon Oxygen 1 1 3 Formalin 40% solution of + formaldehyde CH2O C H O Carbon Hydrogen Oxygen 1 2 1 Gypsum calcium sulfate dihydrate CaSO4⋅2H2O Ca S O H Calcium Sulfur Oxygen Hydrogen 1 1 6 4 Liquid paper* titanium dioxide TiO2 Ti O Titanium Oxygen 1 2 MSG monosodium glutamate C5H8NNaO4 C H N Na O Carbon Hydrogen Nitrogen Sodium Oxygen 5 8 1 1 4 Nutrasweet®* aspartame C14H18N2O5 C H N O Carbon Hydrogen Nitrogen Oxygen 14 18 2 5 Sugar (Table Sugar) sucrose C12H22O11 C H O Carbon Hydrogen Oxygen 12 22 11 Tylenol®* acetaminophen C8H9NO2 C H N O Carbon Hydrogen Nitrogen Oxygen 8 9 1 2 ¶ ¶ From: http://www.ivy-rose.co.uk/Chemistry/GCSE/Common-Chemicals-in-the-Home_Molecular-Formulae.php