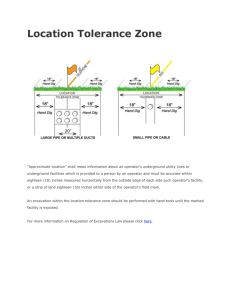
Further Processing Code of Practice
advertisement

Further Processing Code of Practice Part 3: Good Operating Practice Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Prelims Page 1 Prelims Amendment 0 July 2009 Table of Contents Prelims 1 1 Heat Treatment 3 2 Commercial Sterilisation 4 2.1 Purpose and Scope ...................................................................................................4 2.2 Definitions ..................................................................................................................4 2.3 Regulatory Requirements ..........................................................................................6 2.4 Procedures (for compliance) .....................................................................................6 2.5 References: .............................................................................................................28 3 Concentration and Drying 30 3.1 Purpose and Scope .................................................................................................30 3.2 Definitions ................................................................................................................31 3.3 Regulatory Requirements ........................................................................................31 3.4 Procedures (for compliance) ...................................................................................32 3.5 References ..............................................................................................................40 4 Hurdle Technology 42 4.1 Purpose and Scope .................................................................................................42 4.2 Definitions ................................................................................................................42 4.3 Regulatory Requirements ........................................................................................42 4.4 Procedures (for compliance) ...................................................................................43 4.5 References ..............................................................................................................50 5 Smoking 51 5.1 Purpose and Scope .................................................................................................51 5.2 Definitions ................................................................................................................52 5.3 Regulatory Requirements ........................................................................................52 5.4 Procedures (for compliance) ...................................................................................52 5.5 References ..............................................................................................................61 6 Acidification 63 6.1 Purpose and Scope .................................................................................................63 6.2 Definitions ................................................................................................................63 6.3 Regulatory Requirements ........................................................................................64 6.4 Procedures (for compliance) ...................................................................................64 Further Processing Code of Practice Part 3: Good Operating Practice 6.5 7 July 2009 Amendment 1 Prelims Page 2 References ..............................................................................................................70 High Pressure Processing 71 7.1 Purpose and Scope .................................................................................................71 7.2 Definitions ................................................................................................................71 7.3 Regulatory Requirements ........................................................................................72 7.4 Procedures (for compliance) ...................................................................................72 7.5 References ..............................................................................................................82 Further Processing Code of Practice Part 3: Good Operating Practice 1 Heat Treatment pending July 2009 Amendment 1 Heat Treatment Page 3 Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 4 2 Commercial Sterilisation 2.1 Purpose and Scope This section contains the requirements for the commercial sterilisation of low acid canned foods. The term ‘canned’ includes rigid, semi-rigid and flexible packaging options. This section does not apply to aseptic processing e.g. Ultra High Temperature processing (UHT). Production of commercially sterile product is dependent on three key conditions: sealing of the container in such a manner that microorganisms cannot re-enter and contaminate sterilised product; application of heat to the product for a time and at a temperature sufficient to produce commercial sterility; proper post-process handling procedures that protect the finished closures from damage, which could cause leakage or post-process contamination. 2.2 Definitions Come up time is the time which elapses between the introduction of steam to the retort and the time the retort reaches the specified processing temperature. Commercial sterilisation means the condition achieved by application of heat, sufficient alone or in combination with other appropriate treatments to render the product free of microorganisms capable of growing under normal non-refrigerated conditions at which the product is likely to be held during distribution and storage. Critical failure in relation to container closures means anything that would affect the subsequent integrity of the container. F0 is a measure of the amount of lethal heat which results from a specified thermal process (usually measured at the point of lowest lethality in the container). The number is the lethal effect equivalent to the number of minutes at 121.1°C when assuming instantaneous heating and cooling and a z value of 10°C. Flexible container is a container where its shape or contour is affected by the enclosed product (e.g. retort pouches). Headspace means the volume in a container not occupied by the product. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 5 Heat-penetration tests means the scientific experiments conducted to determine heating and cooling behaviour of a product/package combination, processed in a specific retort system, in order to establish safe thermal processes that will result in commercially sterile product or to evaluate process deviations. Heat processing time means the time that the sealed containers are held at the specified processing temperature. Hermetically sealed means air tight, completely sealed and impermeable to gas. Initial temperature (IT) means the average temperature of the contents of the coldest container to be retorted when the heat processing begins, i.e. at the start of the come-up time and is usually the first container to be prepared for processing. Leaker means a sealed and heat processed container which has a defect that allows the passage of water, gas or microbes into the container. Lethality means the effect of exposure to time and temperature transformed mathematically to give a measure of the sterilisation achieved (summed values usually being expressed as F or F0). Minimum initial temperature means the lowest initial product temperature in a container specified in the scheduled process that the process has been developed for. Operator in relation to an animal product business means the owner or other person in control of the business. Qualified canner means a person who meets the competency specification set out in clause 25(2) of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Retort means a pressure vessel, designed for heat processing product packed in hermetically sealed containers. Rigid container is a container where its shape or contour is neither affected by the enclosed product nor deformed by an external mechanical pressure of up to 0.7kg/cm2 (10psig) (e.g. cans, glass jars). Saturated steam means pure steam in equilibrium with water at the same temperature. Under these conditions, the temperature of the steam is entirely dependent on its pressure. Scheduled process means the thermal process alone or in combination with critical factors, and verified by an independent qualified canner, for a given product formulation, container type and size and thermal processing system to achieve commercial sterility of the product. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 6 Semi-rigid container is a container where its shape or contour is not affected by the enclosed product under normal atmospheric temperature and pressure but can be deformed by an external mechanical pressure of less than 0.7kg/cm2 (10 psig) (e.g. tetra-bricks, pottles). Temperature-distribution study are scientific experiments conducted to determine the time, temperature or other parameters that must be met to ensure uniform temperature is established in the retort system. Vacuum means the negative internal pressure in the container produced during the seaming process. Venting means flushing air out of steam retorts at the beginning of a heat process. It is done by allowing large volumes of steam to flow through the retort to drive and carry air out through open vents in the retort. z value is a measure of the temperature resistance of the target microorganism, i.e. the temperature change required to effect a tenfold change in the rate of microbial destruction. 2.3 Regulatory Requirements Refer to Part 2 for the specific legal requirements that apply to commercial sterilisation. 2.4 Procedures (for compliance) 2.4.1 General The operator must document procedures for the commercial sterilisation of low acid canned products which complies with the principles detailed in one of the following codes: a. the Recommended International Code of Hygiene Practice for Low-acid and Acidified Low-acid Canned Foods, as published by the Codex Alimentarius Commission: b. the United States Food and Drug Administration Requirements for Thermally Processed Low-acid Foods Packaged in Hermetically Sealed Containers, as contained in 21 CFR Part 113, and Acidified Foods as contained in 21 CFR Part 114, as appropriate: c. the Code of Practice for the Thermal Processing of Low-acid Canned Food, as published by the Australian National Health and Medical Research Council. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.2 July 2009 Amendment 1 Commercial Sterilisation Page 7 Outcome of Commercial Sterilisation 2.4.2.1 The operator must ensure that an F0 of 3 or greater is achieved in the product, unless full scientific justification for a lower F0 has been validated in the risk management programme. The operator must ensure the inhibition or inactivation of spoilage organisms capable of growing under normal non-refrigerated conditions at which the product is likely to be held during distribution and storage during its shelf life. 2.4.2.2 The operator must document any regulatory limits that are appropriate for the product. There are currently no regulatory limits for commercially sterilised products. 2.4.2.3 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and are not defined in legislation. Operator-defined limits may be expressed as a: i. product requirement, e.g. microbiological limit; ii. process parameter, e.g. minimum time temperature combination; iii. performance criteria, e.g. 12D reduction in pathogen. They may be taken from sources such as reputable COPs, peer-reviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from the Operator’s own trials and experiments. The minimum F0 to be achieved for each commercially sterile product needs to be specified as an operator defined limit in the risk management programme. 2.4.3 2.4.3.1 Competency Personnel responsible for supervising the operators of processing systems, retorts, aseptic processing systems, product formulation systems and performing container closure inspections under the Animal Products Act must meet the competency requirements of clause 25(1)(b) of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Commercial sterilisation operations cannot occur unless there is a person on site with one of the specified qualifications. The operator must ensure that there is sufficient trained staff available to Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 8 cover for illnesses, holidays, resignations etc to ensure that production can continue at these times. 2.4.3.2 Personnel supervising the development of scheduled processes (including equipment commissioning) must meet the competency requirements of clause 25(2) of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. 2.4.3.3 Personnel responsible for checking and signing off scheduled processes prior to release for commercial production must meet the competency requirements of clause 25(2) of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. 2.4.3.4 Companies operating under the Food Act must ensure that they comply with the competency requirements in regulation 13 of the Food Safety Regulations 2002. Manufacture of low-acid canned food must be supervised. 2.4.4 Equipment Details of equipment requirements are specified in the following codes. As mentioned in section 2.4.1, the operator must comply with one of these codes: a. the Recommended International Code of Hygiene Practice for Low-acid and Acidified Low-acid Canned Foods, as published by the Codex Alimentarius Commission: b. the United States Food and Drug Administration Requirements for Thermally Processed Low-acid Foods Packaged in Hermetically Sealed Containers, as contained in 21 CFR Part 113, and Acidified Foods as contained in 21 CFR Part 114, as appropriate: c. the Code of Practice for the Thermal Processing of Low-acid Canned Food, as published by the Australian National Health and Medical Research Council. 2.4.4.1 Retorts must be equipped with: 1. a pressure gauge; 2. an independent standardised mercury-in-glass thermometer (MIG) or alternative temperature measuring device with at least an equal accuracy e.g. resistancetemperature devices (RTDs) that has been: Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 9 a. calibrated by a suitably skilled person; b. calibrated annually (as a minimum) for MIGs and at a frequency recommended by the manufacturer for alternative devices (in most cases this is much more frequent than for MIGs). c. repaired or replaced if the deviation from the standard is more than 0.5°C and in the case of a MIG, if the mercury column is divided; 3. an automatic temperature recording device adjusted to read no higher than the known accurate independent temperature measuring device; An accurate, readable timing device must be readily visible in the retorting room. 2.4.4.2 All equipment used for critical measurements must meet clause 28 of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004 which relates to calibration. 2.4.5 2.4.5.1 Raw Materials Raw materials must comply with the requirements of the Food Standards Code. 2.4.5.2 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. 2.4.5.3 During the development of new products or the modification of existing formulations personnel must consider the impact of ingredient microbiological loading and tailor the process appropriately. 2.4.5.4 The operator must have good systems in place to ensure that ingredients are not changed in a formulation (including supplier, type or addition rate) without input from a suitably skilled person. The initial microbiological loading of raw materials greatly influences the intensity of the thermal process required. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.6 2.4.6.1 July 2009 Amendment 1 Commercial Sterilisation Page 10 Container Materials and Filling All packaging must comply with clause 30 of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004 and the operator must have documented evidence of compliance. 2.4.6.2 Packaging must be obtained from reputable suppliers whom are able to provide competent technical support with regard to use. The supplier must provide written specifications for can or other packaging sealing parameters. The intended use of the packaging must comply with the manufacturer’s specification and must be appropriate to the filling and sealing equipment used. 2.4.6.3 Before filling, packaging must be clean and sound. 2.4.6.4 Cleaning of rigid containers may be achieved by: a. inverting the containers to dump out dust and foreign matter, where appropriate; and b. blasting the inside of the containers to loosen and remove dust and foreign matter, using water (including steam), air, or vacuum. Semi-rigid containers should not be washed prior to use unless effective drying is possible, as water in the sealing area may reduce the seal reliability. 2.4.6.5 Flexible containers should require no cleaning before use and must be stored in a manner which prevents contamination. 2.4.6.6 During container filling, containers must be: a. filled with correctly formulated and prepared product, that is maintained at or above the minimum initial temperature as per the scheduled process; b. filled with the correct weight of product as per the scheduled process; c. filled to the correct level, i.e. sufficient headspace (as applicable) must be left and sufficient vacuum produced by the methods specified in the scheduled process. d. for flexible and some semi-rigid packaging, filled to ensure that the maximum residual air for the scheduled process is not exceeded. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 11 A scheduled process is the validated thermal process together with the critical factors for a given product formulation, container type and size and thermal processing system that when applied, will result in commercial sterilisation of a product. The concepts referred to in this section such as the scheduled process and minimum initial temperature (IT) are addressed in more detail in subsequent sections. 2.4.7 Container Closure and Handling 2.4.7.1 All containers must be hermetically sealed. 2.4.7.2 During closure every container must be dated with the date of packing, e.g. at the time of closure or immediately after. It is desirable to state the retort load and/or time period. A company may date a day’s production, instead of individual retort load or time period, on the understanding that the whole day’s production is involved if a rejection occurs. 2.4.7.3 If processing with glass special consideration must be given to line design and operation to minimise the impact of any breakages. A procedure must be developed to deal with glass breakages when they occur. 2.4.7.4 Procedures must be developed to deal with dropped or damaged packaged product that has not been commercially sterilised to ensure that it is not mistaken for acceptable product and processed as such. 2.4.8 2.4.8.1 Container Closure Evaluation Container closure evaluation must be conducted with sufficient frequency to ensure the adequacy of the hermetic seal. The intervals must be appropriate to the equipment, line speed and container type. 2.4.8.2 Containers must be assessed during production and any critical failures must result in appropriate corrective actions being taken immediately, which is likely to include the ceasing of container closure on the affected piece of equipment until control is restored. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.8.3 July 2009 Amendment 1 Commercial Sterilisation Page 12 In the event of critical failure, all products produced since the last in- specification check must be isolated and assessed by suitably skilled staff and appropriate disposition made (to release with or without restriction, rework or dispose of). 2.4.9 Can Closure Evaluation 2.4.9.1 Closures must be evaluated at the following frequencies for cans from each head of each filling machine: a. Visual examination, including measurement of seams, at least every 30 minutes; and b. Tear down examinations or seam cross section analysis by seam scope or projector (sufficient to measure all critical parameters) at intervals not exceeding 4 hours. c. Visual examinations must also be carried out: i. at start-up; ii. after work has been done on the seamer; iii. after a prolonged shutdown; iv. after a seamer jam-up; and v. after changing container size or body and end material. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.10 July 2009 Amendment 1 Commercial Sterilisation Page 13 Semi-rigid and Flexible Container Closure Evaluation The evaluation of seal quality may differ between package designs, construction and sealing methods and requires thorough research. The following documents provide useful guidance (refer to section 2.5 for full reference details). Canned Foods. Guidelines for closure evaluation are contained in Principles of Thermal Process Control, Acidification and Container Closure Evaluation. CCFRA. (1991). The Shelf- Stable Packaging of Thermally Processed Foods in Semi-Rigid Plastic Barrier Containers – A guideline to good manufacturing practises. Guideline No 31. CCFRA. (1995). Protocols for Performance Testing of Shelf Stable Heat Sealable Containers. Guideline No. 7. CCFRA. (2006). Guidelines on Good Manufacturing Practice for heat Processed Flexible Packaging. Guideline No. 50. CFIA. 2002. Flexible Retort Pouch Defects Identification and Classification Manual. 2.4.11 Semi-rigid Containers 2.4.11.1 Operators must determine the tests to be done through consultation with the packaging and sealing equipment suppliers. The following is guidance only: Seals should be evaluated as a minimum at the following frequencies for containers from each head of each filling machine: visual examination for defects at least every 15 or 30 minutes; and other tests at a recommended frequency of four hours of continuous production, (also see the table following this box). Visual examinations and destructive tests should also be carried out: at start-up; after work has been done on the sealer; after a prolonged shutdown; July 2009 Amendment 1 Commercial Sterilisation Further Processing Code of Practice Part 3: Good Operating Practice after a sealer jam-up; and after a splice or lot change of the body or lid material. Test method Plastic with double seamed metal end Burst test Semi-rigid sealed lid Page 14 Paperboard X Compression, squeeze test X Dye penetration test X X Electrolytic X X Proximity tester X Seam scope projection X Sound X X Tensile (peel) test X Vacuum testing X X Source: Canned Foods. Principles of Thermal Process Control, Acidification and Container Closure Evaluation 2.4.12 2.4.12.1 Flexible Containers Operators should determine the tests to be done through consultation with the packaging and sealing equipment suppliers. The following is guidance only: Seals should be evaluated as a minimum at the following frequencies for pouches from each fill tube or sealing lane. All seals formed by the operator must be tested: if possible, 100% inspection of seals; visual examination of seals for completeness and a squeeze test at least every 30 minutes; and other tests at a recommended frequency of four hours of continuous production e.g. burst test, tensile (peel) test, drop test, dye penetration test. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 15 Visual examinations and destructive tests should also to be carried out: at start-up; after work has been done on the sealer; after a prolonged shutdown; after a sealer jam-up; for web fed systems, after every splice; and after changes to the sealing temperature, pressure or dwell time. 2.4.13 Glass jars There are many different designs of glass jar closures. The manufacturer’s recommendations should be followed as well as the guidelines for closure evaluation contained in “Principles of Thermal Process Control, Acidification and Container Closure Evaluation”. 2.4.14 Temperature Distribution Studies Before a retort is brought into service and any heat penetration work undertaken, temperature distribution studies must be carried out to determine the slowest heating point in the vessel, i.e. the point that is slowest to reach the scheduled processing temperature, and to confirm that the range of temperatures experienced throughout the retort is within prescribed limits. Temperature distribution studies are also used to verify the adequacy of the venting operation for retorts heated solely by saturated steam. 2.4.14.1 Temperature distribution studies must be carried out to determine the distribution of temperatures throughout the loaded retort under the most demanding normal operating conditions, as appropriate to the nature of the retort operation; and to identify the location of the lowest temperature or uneven temperature distribution in the retort. 2.4.14.2 Where required, temperature distribution studies must be carried out to establish an adequate venting schedule on each container size and loading configuration, or the venting schedule for the most difficult container size and loading configuration to vent must be determined and used as the standard. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.14.3 July 2009 Amendment 1 Commercial Sterilisation Page 16 The qualified canner must identify all factors critical to temperature distribution within the retort. 2.4.14.4 Temperature distribution studies must be conducted whenever there are changes to the retort plumbing, container arrangement, introduction of dividers or anything else that could negatively impact on heat transfer in the retort and as a minimum be repeated every 2-3 years. 2.4.14.5 The retort installation must be inspected annually by the operator to ensure no changes have occurred. 2.4.14.6 Documented evidence of the temperature distribution studies must be available. The following references may provide useful information when conducting temperature distribution studies (refer to section 2.5 for full reference details): Adams, H.W. and Hardt-English, P.K. (1990). Determining Temperature Distribution in Cascading Water Retorts. Food Technology 44(12) 110-112. CCFRA. (1997). Guideline for Establishing Heat Distribution in Batch Overpressure Retort Systems. Guideline No. 17. Institute for Thermal Processing Specialists. (2004). Temperature Distribution Protocol for Processing in Steam Still Retorts, Excluding Crateless Retorts. Institute for Thermal Processing Specialists. (2004). Protocol for Conducting Temperature Distribution Studies in Water-Cascade and Water-Spray Retorts Operated in a Still Mode, Including Agitating Systems Operated in the Still Mode. Institute for Thermal Processing Specialists. (2004). Temperature Distribution Protocol for Processing in Steam Still, Water Immersion Retorts, Including Agitating Systems Operated in a Still Mode. Park, D.J., Cabes, W. and Collins, K.M. (1990). Determining Temperature Distribution in Rotary, Full-Immersion, Hot-Water Sterilisers. Food Technology 44(12): 113-118. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.15 July 2009 Amendment 1 Commercial Sterilisation Page 17 Development of a Scheduled Process Heat penetration tests monitor the time/temperature history of the product at the slowest heating point in the container. The results are converted into arbitrary units of lethal heat, are summed over the whole process to give a total lethal effect and are usually expressed as an F0 value. The tests are carried out in the slowest heating area of the retort. 2.4.15.1 The minimum F0 to be achieved for each commercially sterile product must be specified as an operator defined limit in the risk management programme. Consideration should be given to microbial flora, pH, product composition and formulation, levels and types of preservatives, water activity and likely storage temperature when determining appropriate F0s. 2.4.15.2 The following information must be included in the scheduled process. This list is not exhaustive. Qualified canners must use their experience to ensure that all relevant factors are addressed. a. name of the qualified canner that developed the scheduled process; b. date of development; c. name of the qualified canner that independently verified the scheduled process; d. product name, code, type and formulation reference; e. preparation, filling and closure of product (hot fill, cold fill, product arrangement, headspace, pre-cook); f. detailed formulation (including particle size, fat content, % composition, % solids, pH, net weight, consistency or viscosity); g. size and type of the containers; h. minimum initial product temperature; i. all other critical factors in the product that must be controlled and the associated parameters (e.g. maximum product thickness for flexible containers); j. pre-process hold time (minimum and maximum); k. retort(s) the process applies to; l. method of container loading, orientation and where appropriate the speeds; m. venting schedule where appropriate; Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 18 n. come-up time – temperature parameters; o. sterilisation time - temperature parameters; p. overpressure schedule where appropriate; q. cooling procedure, including cooling water temperature; r. heat penetration data; s. test conditions used to design the scheduled process, including the data acquisition system, cold spot location studies (within a container and within retort); t. F0 achieved by the process; u. any other information that is relevant to a particular product and process. 2.4.15.3 The design must be based on the worst case scenario for the given product specifications and equipment capabilities. 2.4.15.4 Heat penetration tests must be validated in the production retort under commercial operating conditions to confirm the adequacy of the process parameters. This includes provisional schedules that are derived from F0 calculations using data from similar processes or from reference material. 2.4.15.5 As a minimum at least 3 confirmatory runs with 3 containers must occur. This may need to be increased in situations where there is unacceptable variation within and between runs as determined by the qualified canner. In some cases, where products are very similar and the qualified canner has a lot of historical data to support their findings, 2 confirmatory runs may be sufficient. In these situations it is recommended that more than 3 containers be tested in each run. 2.4.15.6 The operator must ensure that no changes are made to any critical factor, including product formulation, container size or retorting unless developed and independently verified by a qualified canner, prior to operation. The following references may provide useful information when developing a scheduled process (refer to section 2.5 for full reference details): CCFRA. (1994). Guidelines for the Production of Heat Sterilised/Pasteurised Foods, in Batch Retort Systems Using Full Water Immersion, Raining Water or Steam/Air Mixtures as the Heating Medium (draft). Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 19 CCFRA. (1997). Guidelines for Batch Retort Systems – Full Water Immersion – Raining/Spray Water – Steam/Air. Guideline No 13. CCFRA. (1997). Guidelines for Performing Heat Penetration Trials for Establishing Thermal Processes on Batch Retort Systems. Guideline No. 16. CCFRA. (1997). Guidelines for Performing Heat Penetration Trials for Establishing Thermal Processes in Batch Retort Systems. Guideline No. 16 CFIA. (2002). Protocol for Carrying Out Heat-Penetration Studies. Chapter 13, subject 3. Institute for Thermal Processing Specialists. (2004). Protocol for Carrying Out Heat Penetration Studies. 2.4.16 2.4.16.1 Container Washing When required, to remove adhered organic material, containers should be washed after closure using water sprays or a continuous flow water bath, at a temperature that is adequate to ensure the removal of any product scraps. This water may contain an MAF approved detergent. In the case of cans, extraneous material should not remain on the surface as this will induce corrosion and rust formation. Sealed containers that are dirty should be rinsed to remove protein residues and then washed with hot water and detergent. Washing containers in hot water without pre-rinsing may coagulate soluble proteins making them difficult to remove. 2.4.17 2.4.17.1 Retort Loading To ensure that all containers undergo a thermal process, all retort baskets, trolleys or containers must be marked with temperature change indicators (e.g. cards, strips) which change colour permanently if they are heated to a specific temperature, or other effective means to visually indicate whether or not the product has been retorted. These indicators are not to be used to confirm that heat treatment has been sufficient to fulfil the scheduled process. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.17.2 July 2009 Amendment 1 Commercial Sterilisation Page 20 Retort racks must be capable of holding flexible containers (pouches) so that they do not move during the heat process cycle and so that uniform heating and cooling of all pouches is achieved at the required temperatures. Loose edges of the pouches may overlap but the product inside the pouch must not overlap. 2.4.17.3 Any flexible containers (pouches) that have overlapped or are found to be out of position after the process must be discarded or, if appropriate, reprocessed. 2.4.18 Heat Processing 2.4.18.1 The scheduled process must be followed. 2.4.18.2 The scheduled process, including all parameters critical to the retort operation for the product being retorted must be prominently displayed or obviously readily available to the retort operator. 2.4.18.3 Retort operators must know what actions to take in the event of a process deviation. This may be achieved through training and the provision of written procedures which are readily available to the retort operator. 2.4.18.4 Product must be heat processed promptly. The maximum time from the containers being filled to being processed must be specified, taking into consideration; a. conditions which may permit microbial growth; and b. the production of heat stable toxins; and c. the impact on heat transfer characteristics of the product; The time must not exceed 2 hours unless evidence of an acceptable alternative is available. 2.4.18.5 The IT of the coldest container(s) in a retort load (e.g. usually the first container closed) must be checked and recorded, thoroughly mixing the contents of the container before measuring the temperature; a. For still and discontinuous agitating retorts, by removing the container intended to be loaded first, or the coldest, from those being loaded into the retort. When the retort is loaded, closed, and the steam turned on, the IT of the container set aside is measured. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 21 b. For continuous agitating retorts and hydrostatic retorts, by periodically selecting a container just before it enters the retort. 2.4.18.6 The IT must never be lower than the minimum initial temperature specified in the scheduled process. If the IT is below that specified in the scheduled process and heat processing has started, the heat processing time may be recalculated based on the new IT and the time of retorting extended as calculated, in which case the procedures in section 2.4.24 apply. 2.4.19 Container Cooling 2.4.19.1 Water cooling must take place according to the scheduled process. 2.4.19.2 Cooling water must be of consistently low microbial content, e.g. with an aerobic mesophile count of less than 100 cfu/ml at the end of cooling. In general it is recommended that aerobic mesophile count of cooling water be checked at least weekly and coliforms tested for monthly. Any significant variation from the established limits should be investigated and the level of sampling increased. The presence of coliforms in any sample indicates the need for immediate investigation. 2.4.19.3 Chlorinated cooling water must contain: a. Either a minimum of 5 ppm free residual chlorine at the time of delivery to the retort or the can cooling tank; or b. a detectable amount of free residual chlorine after the retorting process, provided that the detectable chlorine level is measured after each processing batch, or hourly for continuous operations, at the point where the cooling water exits from the retort and if the cooling water fails to show a detectable amount of free residual chlorine then the production since last compliant result must be completely reprocessed or disposed. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 22 2.4.19.4 Chlorine must have a water contact time of at least 20 minutes. 2.4.19.5 Retorts that operate using a closed system of re-circulating the same water for both heating and cooling, e.g. "Steriflow", where the water is sterilised as part of the process need not be chlorinated, provided the operator has evidence that the water is fit for cooling the product. 2.4.19.6 Where an alternative method of water treatment is used, it must have an outcome which is at least equivalent to the use of chlorine. 2.4.19.7 Container cooling water may be re-used for further cooling purposes provided the water is treated to meet the full physicochemical and microbiological standards for potable water and is re-chlorinated or otherwise treated to meet the requirements above. 2.4.20 Post Process Treatment Handling 2.4.20.1 Containers must be rapidly cooled to a temperature of 40°C or less after heat sterilisation and dried quickly before handling. 2.4.20.2 Containers may be removed from retorts at temperatures warmer than 40°C, provided there are procedures in place that ensure the cooling, drying of containers and the hygiene of the container environment does not result in the growth of any bacteria in the container. There is an optimum temperature range for the removal of cooled containers. Thermophiles can grow in the range of 35°C-50°C but excess cooling can lead to containers failing to dry and in the case of cans, rust formation and degradation of the container. If cooling failure occurs and containers are removed for cooling at high temperatures, incubation and/or microbiological assessment should be undertaken to determine product disposition options. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.20.3 July 2009 Amendment 1 Commercial Sterilisation Page 23 Containers must not be removed from any retort at a temperature that is likely to result in container distortion as a result of any physical stress, and must be handled in a manner that will prevent damage to the seal area resulting in potential microbial contamination. 2.4.20.4 Handling of hot and wet containers after retorting must be minimised. Manual unloading of wet containers presents a risk of contamination from pathogens that may be transferred from the worker’s hands into the container by micro-leakage. Flexible pouches are much more susceptible to damage than metal cans and need to be handled carefully to avoid damage. 2.4.20.5 All conveyors, tracks, belts and bars which the containers may come in contact with must be thoroughly cleaned and sanitised to ensure no build up of micro organisms. 2.4.20.6 Cans must not be washed after retort cooling. 2.4.20.7 Only single use sterile wipes may be used for wiping containers. 2.4.20.8 Access to container cooling areas must be controlled and minimised. 2.4.20.9 To ensure that the opportunity for contamination is minimised the container cooling environment must be protected from possible sources of contamination. Consideration should be given to air flow, presence of water including condensation and personnel. Affected personnel should be trained in the importance of personal hygiene and behaviour in relation to container cooling areas. Access should be limited to those persons who reasonably need to be present to complete the required tasks. 2.4.20.10 The operator must have a system to monitor the integrity of processed containers (e.g. dud detection equipment, visual inspection programme, teardowns). Defective containers must be rejected. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.21 2.4.21.1 July 2009 Amendment 1 Commercial Sterilisation Page 24 Labelling and Storage Adhesives and labels which do not attract water must be used if the containers could corrode (e.g. rusting of tin-plate). 2.4.21.2 Stored product must be kept dry and not subjected to extremes of temperature and humidity. 2.4.22 Incubation At least one, and preferably two, containers from each retort load should be incubated for 10 days at 37°C. For continuous retorts (chain, carrier, rotational mechanism), take at least one container per elapsed heat processing time. The temperature of the incubator should be recorded periodically using a calibrated independent thermometer. The retort load or lot from which the container was taken should be held in store until satisfactory incubation tests have been completed. Product may be released prior to completion of the incubation tests provided all other records indicate that the product is within specification and it remains under the control of the operator, (i.e. is prevented from entering retail sale) and can be withdrawn efficiently and effectively in the event of an unsatisfactory incubation test results. 2.4.22.1 If an incubation test is unsatisfactory the retort load or lot from which the container(s) came must be disposed of and the controls reviewed. “Unsatisfactory“ includes any deviation from the product specification such as gas formation, vacuum change, can leakages or pH changes at levels which are considered unacceptable for that product as defined by the qualified canner. 2.4.23 Records Different sterilising systems have different critical factors which must be checked and records made. The different types of sterilising systems and the operation of these are detailed in “Principles of Thermal Process Control, Acidification and Container Close Evaluation”. Also detailed in the same publication are the requirements for the records to be generated to demonstrate control of the process. Further Processing Code of Practice Part 3: Good Operating Practice 2.4.23.1 July 2009 Amendment 1 Commercial Sterilisation Page 25 Permanent records must be kept for each retort load/operation and all test results must be recorded. 2.4.23.2 The information to be recorded at the time of processing, as appropriate to the nature of the process and equipment includes: a. establishment; b. date; c. retort identification; d. product name or other identification; e. product code; f. container size; g. approximate number of containers: h. product initial temperature (IT); i. time that steam was turned on for a batch process; j. time that vents were closed, where appropriate; k. temperature in the retort when the vents were closed, where appropriate; l. time that process was started and in the case of a continuous process, the time the first container enters the retort; m. processing temperature; n. processing time; o. temperature reading from the independent temperature measuring device and from the temperature recorder/controller taken at the same time at least once during the process, and in the case of a continuous operation, at least every hour; p. pressure (if appropriate); q. speed (chain, carrier, rotational) checked and recorded at the start of operations, and at least once per load or at least once every 4 hours during continuous operations, or if the speed is changed; or may be continuously recorded; r. time steam was turned off and in the case of a continuous process, the time the last container leaves the retort; Further Processing Code of Practice Part 3: Good Operating Practice s. water cooling time; t. chlorine (or other sanitiser) levels; July 2009 Amendment 1 Commercial Sterilisation Page 26 u. seam check results; v. cross reference to the automatic temperature record (record identifies retort number, date, product, batch or lot, retort operator's name and reviewer's name); w. all other critical factors specified in the scheduled process, at a frequency sufficient to ensure that they remain within the specified limits; x. retort operator's signature or other means of providing sign off. 2.4.23.3 Processing records, including product specification and container closure evaluations must be verified within 24 hours (1 working day). In the event of process deviations see section 2.4.24. The time within which processing records must be verified may be extended to 36 hours if an event occurs which is unforeseen and the required personnel are unavailable. All product must remain under the control of the operator. 2.4.24 2.4.24.1 Deviation from Scheduled Process Deviations from a scheduled process that may result in under processed product must be: a. Addressed during processing by adjusting the time and/or temperature of the heat process during the process to ensure the product is made commercially sterile; or b. Addressed after processing, either: i. the process is completed without alteration and the process deviation is assessed by a qualified canner; or ii. the product may be immediately reprocessed using the scheduled process. In practice, where product is to be reprocessed, this should occur within hours of the initial process, or the product should be immediately cooled to 0°C and held for a maximum of 2-3 days prior to reprocessing. Heat processing times for the reprocessing of products may vary from the scheduled process. This may be because the partial process that has already been applied may have significantly altered Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 27 the heat transfer characteristics and IT of the product. The qualified canner must take these factors into account when assessing the safety of the applied process and determining product disposition. 2.4.24.2 Any product lot(s) affected by a deviation under 2.4.24.1 must be identified, segregated, and detained pending the outcome of an assessment by a qualified canner. 2.4.24.3 Assessments of all deviations must be based on detailed information of the conditions prevailing during the non-scheduled process and on the heat transfer characteristics of the product, in accordance with procedures adequate to detect any potential hazard to public health. 2.4.24.4 The qualified canner must make a decision regarding the disposition of the product based on the outcome of this assessment. The impact on public health must be the primary consideration in any decision taken. Product may either be fully reprocessed (subject to any processing constraints), released if it is determined that the applied process was sufficient to render the product lot(s) commercially sterile or condemned and suitably disposed of. 2.4.24.5 Where the decision is taken to release the affected product, it must be held pending verification of the safety of the product by a qualified canner who is independent of the original assessment. 2.4.24.6 A full report of the assessment and corrective actions taken must be prepared and must include: a. date and time of deviation; b. retort identification; c. complete description of the nature and scope of the deviation, including processing records; d. description of affected product; e. code and quantity of affected product; f. corrective action taken, including restoration of control, product disposition and prevention of recurrence; g. records of the tests undertaken and any reprocessing records; Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 28 h. the name and signature of the qualified canner who conducted the assessment; and i. where the decision is made to release the product, the name and signature of the independent qualified canner who verified the assessment. 2.5 References: Adams, H. W. and Hardt-English, P.K. (1990). Determining Temperature Distribution in Cascading Water Retorts. Food Technology 44(12) 110-112. Anon. (1995). Canned Foods. Principles of Thermal Process Control, Acidification and Container Closure Evaluation. National Food Processors Association. The Food Processors Institute, Washington D.C. Campden and Chorleywood Food Research Association. (2006). Guidelines on Good Manufacturing Practice for Heat Processed Flexible Packaging. Guideline No. 50. Campden and Chorleywood Food Research Association. (1998). Heat Processing of Low Acid Foods: an Approach for Selection of F0 Requirements. Review 9.65. Campden and Chorleywood Food Research Association. (1997). Guidelines for Batch Retort Systems – Full Water Immersion – Raining/Spray Water – Steam/Air. Guideline No 13. Campden and Chorleywood Food Research Association. (1997). Guidelines for Performing Heat Penetration Trials for Establishing Thermal Processes in Batch Retort Systems. Guideline No. 16. Campden and Chorleywood Food Research Association. (1997). Guidelines for Establishing Heat Distribution in Batch Overpressure Retort Systems. Guideline No. 17. Campden and Chorleywood Food Research Association. (1995). Protocols for Performance Testing of Shelf Stable Heat Sealable Containers. Guideline No. 7. Campden and Chorleywood Food Research Association. (1994). Guidelines for the Production of Heat Sterilised/Pasteurised Foods in Batch Retort Systems Using Full Water Immersion, Raining Water or Steam/Air Mixtures as the Heating Medium (draft). Campden and Chorleywood Food Research Association. (1991). The Shelf- Stable Packaging of Thermally Processed Foods in Semi-Rigid Plastic Barrier Containers – A Guideline to Good Manufacturing Practises. Guideline No 31 Canadian Food Inspection Agency. (2002). Flexible Retort Pouch Defects Identification and Classification Manual. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Commercial Sterilisation Page 29 Canadian Food Inspection Agency. Facilities Inspection Manual, Chapt 5, 6 and 13. Canadian Food Inspection Agency. (2002). Protocol for Carrying Out Heat-Penetration Studies. Chapter 13 subject 3. CODEX. (1933). Recommended International Code of Hygienic Practice for Low Acid and Acidified canned foods CAC/RCP 23-1979, Rev 2. Institute for Thermal Processing Specialists. (2004). Protocol for Conducting Temperature Distribution Studies in Water-Cascade and Water-Spray Retorts Operated in a Still Mode, Including Agitating Systems Operated in the Still Mode. Institute for Thermal Processing Specialists. (2004). Temperature Distribution Protocol for Processing in Steam Still, Water Immersion Retorts, Including Agitating Systems Operated in a Still Mode. Institute for Thermal Processing Specialists. (2004). Temperature Distribution Protocol for Processing in Steam Still Retorts, Excluding Crateless Retorts. Institute for Thermal Processing Specialists. (2004). Protocol for Carrying Out Heat Penetration Studies. Park, D.J., Cabes, W. and Collins, K.M. (1990). Determining Temperature Distribution in Rotary, Full-Immersion, Hot-Water Sterilisers. Food Technology 44(12): 113-118. United Kingdom Dept of Health. (1994). Guidelines for the Safe Production of Heat Preserved Foods. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 30 3 Concentration and Drying 3.1 Purpose and Scope This section contains the requirements for the preservation of products by reducing water activity (aw) through the removal of water, where this mechanism is the key preservation step. Aw is a measure of the water that is available to take part in activities such as microbial growth. Aw is a better measure of food perishability than water content, as water content does not take into account whether that water is available for microbiological growth. Aw may be reduced by: removing water (e.g. evaporation, drying); or binding water 1 (e.g. by the addition of permitted ingredients and additives such as salt, sugars, sorbitol or glycols); or a combination of removing and binding water. Products that are preserved by reducing water activity are commonly categorised as low moisture or intermediate moisture foods. Low moisture foods generally contain up to 25% moisture and have an Aw of less than 0.60, for example jerky or biltong. Intermediate moisture foods generally contain between 15% and 50% moisture and have an Aw between 0.60 and 0.85. (Jay, 2000). An example of an intermediate moisture food is semi-dry salami. The Aw of a product determines the type of microorganisms that may grow. Most bacteria require Aw values above 0.90 for growth. An exception is Staphylococcus aureus which may grow and produce enterotoxins in foods with an Aw as low as 0.84. Generally, at an Aw below 0.9, yeasts and moulds are the organisms most likely to grow. Reducing the Aw inhibits microbial growth but is not necessarily a lethal intervention. Bacteria, yeast, moulds, and some foodborne parasites such as Trichinella spiralis may survive a drying process. Heat resistance of bacteria tends to increase as the Aw is lowered and the degree of resistance depends on the compounds being used to control Aw (Jay, 2000). Microbial 1 The addition of permitted ingredients and additives to preserve a product is covered in section 4, Hurdle technology. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 31 growth may re-commence after rehydration of the product under favourable conditions, e.g. during preparation by the consumer. To control the growth of pathogens in shelf stable products the Aw will need to be reduced to 0.85 or less. In products with an Aw of greater than 0.85, additional preservation methods such as chilling, acidification or additives would be needed. The following table provides an indication of the lower Aw limits for growth of bacteria, yeasts and moulds. Aw Micro-organism 0.90 Most bacteria 0.86 S. aureus toxin production 0.85 Many yeasts 0.84 S. aureus growth 0.80 Most fungi 3.2 Definitions water activity (Aw) is a measure of the water available for microbial growth. It is the ratio of the water vapour pressure of the food (p) to that of pure water (po) at the same temperature: Aw = p/po operator-defined limit means a measurable limit established by a risk management programme operator to manage the fitness for purpose of animal material or animal product regulatory limit means a measurable regulatory requirement that is critical to fitness for intended purpose of animal material or animal product. 3.3 Regulatory Requirements Refer to Part 2 for the specific legal requirements that apply to concentrated and dried products. Further Processing Code of Practice Part 3: Good Operating Practice 3.4 July 2009 Amendment 1 Concentration and Drying Page 32 Procedures (for compliance) Outcome of Concentration or Drying 3.4.1 3.4.1.1 The reduction in Aw by concentration and/or drying and any additional controls (where used) must ensure the inactivation or inhibition of targeted vegetative pathogens (e.g. S. aureus, salmonella, E. coli O157:H7, Listeria monocytogenes) and of viable spores (e.g. Bacilli, Clostridia) as identified during the application of HACCP principles, and must be sufficient to render the product microbiologically safe for its intended shelf life and storage conditions. 3.4.1.2 The operator must document any regulatory limits that are appropriate for the product. A regulatory limit is a measurable limit related to fitness for intended purpose, established by the Director-General. Regulatory limits may be expressed as a: i. product requirement, e.g. microbiological limit such as 0 cfu/gm Listeria monocytogenes in packaged cooked cured/salted meat; ii. process parameter, e.g. drying regime; iii. performance criteria, e.g. reduction in pathogen by 2 log. Regulatory limits are stated in the legislation e.g. the Food Standards Code, Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Currently there are few regulatory limits. 3.4.1.3 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and that are not defined in legislation. Operator-defined limits may be categorised into the three types as described for regulatory limits. They may be taken from sources such as reputable COPs, peerreviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from their own trials and experiments. Examples of operator-defined limits could include the Aw of a jerky product, meat extract or Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 33 powder, microbiological criteria for a product that is not currently addressed within the Food Standards Code, a required log reduction of a specified pathogen to be achieved by the process, or drying or concentration processing parameters. In setting an operator-defined limit the operator should consider its intended use, the intended consumer and handling it is likely to receive after it has been released for sale. 3.4.2 3.4.2.1 Raw Materials Raw materials must comply with the requirements of the Food Standards Code. 3.4.2.2 Raw materials must be of an appropriate microbiological status. The operator should have knowledge of the microbiological status of raw materials. This information may be obtained from certificates of analysis from suppliers, from the National Microbiological Database for some meat types, by testing the raw materials, or using information gained from a previous processing step (e.g. if the raw material is a cooked product). The capability of the process, intended use of the product and any further processing and/or preparation prior to consumption should be considered when determining the microbiological criteria for raw materials. Some products may be produced by a drying process that only inhibits the growth of bacteria and may be consumed without any further treatment or preparation that would result in a reduction in levels of bacteria. In some cases the method of preparation by the consumer may even promote microbial growth. 3.4.2.3 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. 3.4.3 3.4.3.1 Development and Validation of the Process The operator must have evidence to support and justify the selection of all operator-defined limits. Further Processing Code of Practice Part 3: Good Operating Practice 3.4.3.2 July 2009 Amendment 1 Concentration and Drying Page 34 The operator must establish a drying or concentration process that will result in product that will meet any regulatory limits and/or operator-defined limits. 3.4.3.3 Drying or concentration processes must be developed and validated by a suitably skilled person, and revalidated by a suitably skilled person whenever there is a change to the process or product that would impact on its safety. It is important that people who are responsible for validation have a good working knowledge of the factors that are critical to drying or concentration operations and the importance of ensuring that the required Aw is attained throughout the product and maintained for the duration of its shelf life. The suitably skilled person will also need to have good knowledge of food microbiology and the growth parameters for the microorganisms of concern. It is the responsibility of the operator to ensure that people with the appropriate knowledge and skills are used. 3.4.3.4 The rate and conditions of drying or concentration must be such that undesirable microbial growth and contamination of the product is minimised. 3.4.3.5 Records of the validation must be retained. The complexity of the validation of the process will depend on the nature of the operation, the identified hazards, the control measures involved, and the availability of established process parameters or criteria. For example, for a simple or standard process, validation may only require demonstration that the process is operated in accordance with validated process parameters and/or criteria obtained from a reputable agency or research institute, or published in a scientific journal and/or that the product consistently meets the required Aw. For a novel or more complex process, the operator may need to establish their own process parameters and/or criteria, which are capable of producing in product that is fit for its intended purpose, and then provide evidence of achievement of that process. In a few cases challenge trials may be necessary. 3.4.3.6 Drying or concentration processes must be validated considering all relevant factors, such as: a. micro-flora and microbial load of raw materials; b. potential for microbiological growth; c. product formulation (including moisture content, concentration, viscosity, product shape and geometry, particle size); Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 35 d. processing regime (including feed rate, loading (to ensure even drying and to avoid overloading which could cause slow drying and promote rapid microbial growth), residence time, temperature, air velocity, vacuum, humidity); e. dryer capability e.g. sufficient for intended throughput; f. case hardening (this may occur in products of a reasonable size or thickness if the drying parameters fluctuate, or the drying procedure is not appropriate for the product, leading to surface drying while the centre remains moist. Additional drying is often ineffectual as the moisture is sealed into the product); g. any other preservation steps to be applied to the product and associated processing parameters (e.g. heat treatment, refrigeration); h. Aw of the final product (this may be routinely verified by measurement of moisture content, solids content, density, brix or salt content etc but needs to be initially validated against Aw); Solids content, density, refractive index or other product parameters may be used to estimate the Aw of a product, but the correlation between the parameter and Aw must be established for that product. The correlation may be established by measuring the parameters in samples of the finished product and then having the Aw analysed e.g. by a laboratory. Provided that the formulation and processing parameters don’t change, the correlation between Aw and the parameter should remain constant. a. handling; b. packaging and storage conditions of finished product; c. any further processing or preparation by the consumer. Process types Concentration by evaporation Concentration by evaporation is used to remove water from high moisture products producing concentrated liquids (e.g. concentrates, broths, stocks) or intermediaries for further drying. The process generally involves feeding pre-heated product into an evaporator where steam condensing on one side of the heating surface (calandria) provides the heat for evaporation of water from the product surface. The vapour is removed and condensed. Condensers, vacuum pumps, or steam ejectors can be used to create a vacuum above the liquid which reduces the boiling point and Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 36 product damage. Drying Drying usually involves placing product in a stream of heated air, or in contact with a heated surface. The objective in drying is to ensure that the vapour pressure of the process medium (normally air) is lower than the vapour pressure of the food. Variability in temperature, air velocity or other parameters throughout a drier could result in different drying rates. Surveys of the final moisture content of product throughout a drier should be used to detect the slowest drying area. If one or more locations emerge that consistently dry more slowly, these locations should be used for routine testing. All drying variables that could impact on the slowest drying area(s) need to be taken into account when determining these locations. It is important with all drying operations that the drying end point is accurately identified and that no damp spots remain. 3.4.3.7 The operator must take sufficient samples of the final product to verify that the required final Aw has been met throughout. The number of samples to be tested will depend on the operator’s knowledge of the process, the consistency of the operation and the variability throughout the dryer. Where product does not meet the validated product criteria it must be returned to the dryer for further drying. Process types Freeze drying The mechanism of freeze drying involves sublimation of water vapour directly from ice. The vacuum system removes the vapours, thus maintaining the vapour pressure differential. The final moisture content of freeze dried products may be about 2-8% or have an Aw of 0.10-0.25. (Jay, 2000). Freeze drying operations preserve micro-organisms. The microbial loading of raw materials is very important for this type of operation. In addition to the generic factors listed in 3.4.3.6, for freeze drying the operator should be able to identify the drying end point and whether any ice spots remain in the product. Ice spots are often associated with areas of thicker product loading. If the product is removed from the dryer while ice spots remain and is allowed to defrost, these areas would have an Aw of 1. This could then allow micro-organisms, including any pathogens to grow. Spray drying Spray drying involves the atomisation of liquid feeds into a hot drying medium. The raw material may be a solution, suspension, emulsion or paste. The feed is atomised using a rotating disc or Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 37 nozzle into a large drying chamber. Due to the small size of the droplets a large surface area is available for evaporation and as this occurs rapidly, lower product temperatures and improved quality result. Uniformity of droplet size is important to ensure consistent and uniform drying. Solar drying Solar drying uses solar radiation to heat the air in contact with the product and evaporate the moisture. It can be very difficult to control drying conditions using these traditional techniques, with the possibility of growth of pathogens and spoilage organisms due to the varying and often slow drying rate. This variability in conditions needs to be accounted for during validation. 3.4.3.8 In addition to the generic factors listed in section 3.4.3.6, for solar drying the following factors must be considered: a. worst case drying rate (minimum drying temperature and maximum drying time); b. maximum humidity; c. exclusion of pests, dirt and other contaminants; d. for indirect drying; collector efficiency (heater configuration, air flow rate, spectral properties of the absorber, air barriers, insulation). 3.4.4 3.4.4.1 Implementation The process must be operated in accordance with the validated process and procedures. 3.4.4.2 The process must be verified at a frequency necessary to ensure that the established process and product parameters are consistently being met. 3.4.4.3 Aw, moisture content or any other measurement used for determining the level or availability of water in the product must be made using calibrated instruments (where appropriate), meaningful sample selection and standardised methods of sample preparation. Sample selection is important in concentration and drying operations as there may be large variation throughout a batch or run. The operator needs to have good knowledge of their process to ensure that the wettest samples are selected for testing. Further Processing Code of Practice Part 3: Good Operating Practice 3.4.4.4 July 2009 Amendment 1 Concentration and Drying Page 38 If the drying or concentration step is a CCP, the step must be carried out and/or supervised by appropriately trained personnel. Where a processing step is a CCP, training expectations are heightened. The operator is to ensure that adequate training is provided and records of the training are kept. The training should cover the operation, control and monitoring of that step. 3.4.4.5 3.4.5 3.4.5.1 Records of the process must be retained for each production lot. Post-process Handling Post-process handling, packaging and storage of the dried or concentrated product must be done in a manner that minimises moisture re-absorption and contamination and maintains the fitness for intended purpose of the product. Sources of moisture may include equipment surfaces following cleaning that have not been sufficiently dried, moisture within packaging (particularly where packaging has been cleaned with water prior to filling), or splash. During processing, sources of water sources should be minimised. 3.4.5.2 Cross contamination between raw and processed products must be minimised. This is of particular importance when product is loaded and unloaded into the same processing area. Ideally, any further processing or handling should occur in dedicated areas (i.e. separate room) which are physically separated from areas where raw materials are handled, using dedicated equipment. 3.4.5.3 During post treatment operations involving exposed product, access of persons from other incompatible areas must be restricted and an appropriate hygiene routine completed. 3.4.6 3.4.6.1 Packaging and Labelling Packaging must be of an appropriate composition for its intended use, storage times and conditions and method of product preparation by the consumer. Packaging which is used for a specific purpose such as modified atmosphere packaging or that is Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Page 39 to remain in place during cooking must be of an appropriate composition for its intended use, storage conditions and method of product preparation by the consumer. 3.4.6.2 Where necessary to ensure the safety of the product, systems must be put in place to check the packaging seal or closure integrity. 3.4.6.3 Any material that is used for sealing product such as metal clips, must be controlled in a manner that will ensure that it is not be a source of physical contamination to the product. 3.4.6.4 Product must be labelled in accordance with the requirements of the Food Standards Code, including any “Directions for Storage and Use” where specific methods of preparation are needed prior to consumption to ensure its safety. 3.4.7 3.4.7.1 Storage The product must be stored under conditions that minimises deterioration and will maintain its fitness for intended purpose for the duration of the shelf life. 3.4.8 3.4.8.1 Deviation from the Validated Process Action must be taken if there is a deviation from the critical parameters that results a final product that does not meet the validated process or product parameters, or would otherwise affect the safety of the product. 3.4.8.2 Any product from section 3.4.8.1 must be identified and segregated until its safety is assessed by a suitably skilled person. 3.4.8.3 A record of the assessment and corrective actions taken must be prepared by the suitably skilled person. The record should be appropriate to the nature of the deviation and should include: a. date and time of deviation; b. equipment identification, where appropriate; Further Processing Code of Practice Part 3: Good Operating Practice c. July 2009 Amendment 1 Concentration and Drying Page 40 description of the nature and scope of the deviation; d. description of affected product, including code and quantity; e. corrective action taken, including restoration of control, product disposition and prevention of recurrence; f. records of any tests undertaken; and g. the name and signature of the suitably skilled person. 3.4.9 Shelf-life 3.4.9.1 The shelf-life must be determined as part of the process development. 3.4.9.2 The operator must demonstrate adequate verification of the shelf-life. For further information on determining shelf life expectancy refer to the MAF document: A Guide to Calculating the Shelf Life of Foods. (460 KB PDF). 3.5 References Brennan, J. G. (2006). Food Processing Handbook. Wiley-VCH Verlag GmbH and Co. Germany. Fennema, O. R. (1985). Food Chemistry. Marcel Dekker Inc, New York, USA. 2nd Ed. Jay, J. M. (2000). Modern Food Microbiology. Aspen Publishers Inc, Maryland, USA. 6th Ed. MAF Food Assurance Authority. (1999). A Guide to HACCP Systems in the Meat Industry. Appendix X.6: Manufacture of Beef Jerky: Generic HACCP Plan for Manufacture of Beef Jerky. Amendment 6. Mujumdar, A. S. (2004). Dehydration of Products of Biological Origin. Science Publishers Inc, Enfield, NH, USA. Mujumdar, A. S. (1995). Handbook of Industrial Drying. Marcel Dekker Inc, New York, USA. Nath, S. and Satpathy, G. R. (1998). A Systematic Approach for Investigation of Spray Drying Processes. Drying Technology 16(6), 1173-1193. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Concentration and Drying Varnam, A. H. and Sutherland, J. P. (1995). Meat and Meat Products. Technology, Chemistry and Microbiology. Chapman and Hall. UK. Page 41 Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Hurdle Technology Page 42 4 Hurdle Technology 4.1 Purpose and Scope Hurdle technology is the application of selected processing techniques in combination to preserve a product. Hurdle technology acts by exhausting bacterial cells as each hurdle that is applied requires the cells to use energy to maintain their internal pH, osmotic pressure and keep toxic molecules out. Traditional hurdles include the control of water activity (Aw), acidity (pH), temperature, redox potential (Eh) and the addition of preservatives or starter cultures. Hurdle technology enables the creation of minimally processed products with the desired sensory and nutritional properties which are safe and stable. It is considered a “gentle” means of preserving a product, as the degree to which each hurdle is applied is minimised with the intent of achieving the best quality product. Examples of foods preserved by hurdle technology include: Shelf stable salamis: preserved using a combination of acid (chemical or starter culture), water activity, lowered Eh (oxygen scrubbing bacteria), salt, nitrite and occasionally smoke. Cold smoked salmon: preserved using a combination of pH, salt and the active ingredients in smoke (e.g. isoeugenol). 4.2 Definitions operator-defined limit means a measurable limit established by a risk management programme operator to manage the fitness for purpose of animal material or animal product regulatory limit means a measurable regulatory requirement that is critical to fitness for intended purpose of animal material or animal product 4.3 Regulatory Requirements Refer to Part 2 for the specific legal requirements that apply to hurdle technology products. Further Processing Code of Practice Part 3: Good Operating Practice 4.4 July 2009 Amendment 1 Hurdle Technology Page 43 Procedures (for compliance) Outcome of Application of Hurdle Technology 4.4.1 The operator must ensure the inhibition or elimination of identified vegetative pathogens (e.g. salmonella, Listeria, S. aureus) and of viable spores (e.g. Bacilli, Clostridia) as determined during the application of HACCP principles, and the process must be sufficient to render the product microbiologically acceptable for its intended method of storage, intended purpose and shelf life. 4.4.1.1 The operator must document any regulatory limits that are appropriate for the product. A regulatory limit is a measurable limit related to fitness for intended purpose, established by the Director-General. Regulatory limits may be expressed as a: iv. product requirement, e.g. microbiological limit such as 0 cfu/gm Listeria monocytogenes in packaged cooked cured/salted meat; v. process parameters; vi. performance criteria, e.g. reduction in pathogen by 2 log. Regulatory limits are stated in the legislation e.g. the Food Standards Code, Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Currently there are few regulatory limits. 4.4.1.2 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and are not defined in legislation. Operator-defined limits may be categorised into the three types as described for regulatory limits. They may be taken from sources such as reputable COPs, peerreviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from their own trials and experiments. Examples of operator-defined limits could include the final pH or Aw of a product, microbiological criteria for a product that is not currently addressed within the Food Standards Code, or a required log reduction of a specified pathogen to be achieved by the process. In setting an operator-defined limit the operator should consider its intended use, the intended consumer and handling it is likely Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Hurdle Technology Page 44 to receive after it has been released for sale. 4.4.2 Raw Materials 4.4.2.1 Raw materials must comply with the requirements of the Food Standards Code. 4.4.2.2 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. The initial microbiological loading (including spore loading) of the raw material greatly influences the intensity of the hurdles needed for the safety of the product. Written ingredient specifications should be agreed with suppliers wherever possible and the implications of changing ingredient suppliers or specifications must be understood by those with responsibility for purchasing and product development. 4.4.2.3 Development and Validation of the Process 4.4.2.4 The operator must have evidence to support and justify the selection of all operator-defined limits. 4.4.2.5 The operator must establish a process that will result in product that will meet any regulatory limits and operator-defined limits. 4.4.2.6 Hurdle technology processes must be developed and validated by a suitably skilled person, and revalidated by a suitably skilled person whenever there is a change to the process or product that would impact on its safety. It is important that persons who are responsible for the development and validation work have a good working knowledge of the factors that are critical to the application of hurdle technology. A suitably skilled person may be someone who has a good knowledge of food microbiology and the types and intensity of hurdles needed to ensure a safe and stable product, and the types of ingredients or processes to establish the hurdles taking into account the technical, legal, sensory and nutritional limitations. It is the responsibility of the operator to ensure that people with the appropriate knowledge and skills are used. Further Processing Code of Practice Part 3: Good Operating Practice 4.4.2.7 July 2009 Amendment 1 Hurdle Technology Page 45 The operator must validate of all hurdle technology processes and keep records of the validation. The only definitive means of validating hurdle technology products is through challenge studies. It is important that the target microorganisms used for the validation work have been thoroughly researched. When using hurdle technology the effect of each hurdle on product safety and shelf life must be considered to ensure that no unexpected outcomes occur. For example, a certain type of modified atmosphere might inhibit the growth of spoilage organisms in refrigerated food which could otherwise inhibit toxin production or act as an indicator of poor storage conditions. Therefore an extension of the product's shelf life may lead to the growth of pathogenic microorganisms without any signs of spoilage. Examples of hurdles: pH or acidity: the specific acid used is important, organic acids are the most effective (e.g. acetic and lactic acids). Suitably skilled persons need to be aware of the ability for acid adaptation by micro-organisms and increased survival rates. Eh or redox potential: microbial growth in foods reduces the Eh as oxygen is scavenged. Decreasing the pH results in an increase in Eh. Aw or water activity: is lowered by the removal of water, addition of solutes or binding of water. Where solutes are used, the solute selected influences the minimum Aw for growth for particular organisms. Heating: non-lethal or lethal Competitive micro flora Antimicrobials: naturally occurring antimicrobials include eugenol (from cloves), allicin (from garlic), thymol (from sage) and bacteriocins (e.g. nisin). other chemical anti-microbials include nitrate/nitrites, sulphur dioxide, sorbic acid, benzoic acid and smoke. Active packaging: include absorbers, scavengers, scrubbers, gas emitters, desiccants and antimicrobial coatings. Novel technologies: include high hydrostatic pressure and high density pulsed electric fields. Further Processing Code of Practice Part 3: Good Operating Practice 4.4.2.8 July 2009 Amendment 1 Hurdle Technology Page 46 Hurdle technology processes must be developed and validated considering all relevant factors, such as: a. micro-flora and microbial load of raw materials; b. potential microbiological growth; c. ability for adaptation by microorganisms to the hurdle and increased resistance (e.g. acid adaptation); d. interaction between hurdles (acting synergistically or antagonistically); e. critical process parameters for each hurdle; f. product handling; g. storage conditions of finished product; h. handling and storage conditions of finished product within the supply chain and by the consumer; i. any further preparation by the consumer. 4.4.2.9 During the application of the different hurdles, the critical processing factors described in other sections of this COP must be applied (e.g. heat treatment, drying, acidification). 4.4.2.10 Product trials must be conducted to validate any process parameters derived from any of the following: a. calculations; b. data from similar processes; c. reference material; d. computer modelling programmes. Further Processing Code of Practice Part 3: Good Operating Practice 4.4.3 4.4.3.1 July 2009 Amendment 1 Hurdle Technology Page 47 Implementation The process must be operated in accordance with the validated process and procedures. 4.4.3.2 The process must be verified at a frequency necessary to ensure that the established process and product parameters are consistently being met. 4.4.3.3 Hurdle technology processes must be carried out and/or supervised by appropriately trained personnel. Where several hurdles are required for appropriate control, but each individually does not meet the requirement for a CCP, the operating parameters must be documented for the hurdles used and each hurdle regularly checked, verified and supervised by appropriately trained personnel. If a processing step is a CCP, training expectations are heightened. The operator is to ensure that adequate training is provided and records of the training are kept. The training should cover the operation, control and monitoring of that step. 4.4.3.4 4.4.4 4.4.4.1 Records of the process must be retained for each production lot. Post-process Handling Product handling must be performed so that recontamination is prevented and pathogen growth is minimised. 4.4.4.2 During post treatment operations involving exposed product, access of persons from other incompatible areas must be restricted and an appropriate hygiene routine completed. Further Processing Code of Practice Part 3: Good Operating Practice 4.4.5 4.4.5.1 July 2009 Amendment 1 Hurdle Technology Page 48 Packaging and Labelling Packaging must be of an appropriate composition for its intended use, storage times and conditions and method of product preparation by the consumer. Packaging which is used for a specific purpose such as modified atmosphere packaging, frozen storage or that is to remain in place during cooking must be of an appropriate composition for its intended use, storage conditions and method of product preparation by the consumer. 4.4.5.2 Systems must be put in place to check the packaging seal or closure integrity where necessary to ensure the safety of the product. This may include visual or physical testing e.g. complete seal, no cracking or wrinkling, maintenance of vacuum. 4.4.5.3 Any material used for sealing product such as metal clips, must be controlled in a manner that will ensure that it is not be a source of physical contamination to the product. 4.4.5.4 Product must be labelled in accordance with the requirements of the Food Standards Code, including any “Directions for Storage and Use” where specific methods of preparation are needed prior to consumption to ensure its safety. 4.4.6 4.4.6.1 Storage The operator must take all reasonable steps to ensure that products are stored in a manner that will maintain their safety and wholesomeness for the duration of the shelf life. 4.4.7 Deviation from the Validated Process Further Processing Code of Practice Part 3: Good Operating Practice 4.4.7.1 July 2009 Amendment 1 Hurdle Technology Page 49 Action must be taken if there is a deviation from the critical parameters that results in a final product that does not meet the validated process or product parameters, or would otherwise affect the safety of the product. 4.4.7.2 Any product from section 4.4.7.1 must be identified and segregated until its safety is assessed by a suitably skilled person. 4.4.7.3 A record of the assessment and corrective actions taken must be prepared by the suitably skilled person. The record should be appropriate to the nature of the deviation and should include: a. date and time of deviation; b. equipment identification, where appropriate; c. description of the nature and scope of the deviation; d. description of affected product, including code and quantity; e. corrective action taken, including restoration of control, product disposition and prevention of recurrence; f. records of any tests undertaken; and g. the name and signature of the suitably skilled person. 4.4.8 Shelf-life 4.4.8.1 The shelf-life must be determined as part of the process development. 4.4.8.2 The processor must demonstrate adequate verification of the shelf-life. For further information on determining shelf life expectancy refer to the MAF document: A Guide to Calculating the Shelf Life of Foods. (460 KB PDF), available on the MAF website. Further Processing Code of Practice Part 3: Good Operating Practice 4.5 July 2009 Amendment 1 Hurdle Technology Page 50 References Faith N G, Yousef A E and Luchansky J B (1992). Inhibition of Listeria monocytogenes by Liquid Smoke and Isoeugenol, a Phenolic Component Found in Smoke. Journal of Food Safety 12:303-314. Leistner L (2002). New technologies of food preservation in the 21st century. TehnologijaMesa. 43(3/6): 127-145 Leistner L (2000). Basic aspects of food preservation by hurdle technology. Int Journal of Food Micro 55:181-186 Leistner L (2002). Hurdle technology. Control of Food borne Microorganisms. Ed: V K Juneja and J N Sofos. 493-508. Marcel Dekker. New York Leistner L and Gorris LGM (1995). Foods Preservation by Hurdle Technology. Trends in Food Science and Technology. 6: 41-46. Leistner L and Gould G W (2002). Hurdle Technologies: Combination Treatments for Food Stability, Safety and Quality. New York: Kluwer Academic/Plenum Publishers. Thurette J, Membre J-M, Han Ching L, Tailliez R and Catteau M (1998). Behaviour of Listeria spp. in Smoked Fish Products Affected By Liquid Smoke, NaCl Concentration and Temperature. Journal of Food Protection. 61: 1475-1479 Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 51 5 Smoking 5.1 Purpose and Scope This section applies to the smoking of products Smoking is undertaken for a variety of reasons including: preservation; aroma and flavour; colour; creation of new products; formation of protective skin on emulsion-type sausages; and protection from oxidation. Smoke is generally more frequently applied to produce a sensory effect, rather than being relied upon to achieve preservation. In almost every case where smoke is used to assist in preservation, other factors also play a critical role in preservation such as drying, salting, cooking, brining, refrigeration or packaging. When smoking products, pre- and post-smoking handling steps must be well managed to avoid cross contamination and to minimise deterioration. Smoke, either from wood or from smoke extracts contain phenols, alcohol, organic acids, carbonyls, hydrocarbons and gases. Although not fully understood, it is believed that the anti-microbial properties of smoke come from specific chemical components (e.g. formaldehydes, phenols and acids). Smoke extracts contain most of the essential chemical components of wood smoke but in most cases has been stripped of the carcinogenic polycyclic aromatic hydrocarbons (PAH). Traditional smoking processes may be divided into three categories depending on the temperatures used in the smoke chamber, for example: Cold smoking at 18-30°C, but may be as low as 10°C (e.g. salmon (ready-to-eat and those that must be cooked), salamis); Semi-warm smoking at around 40°C (e.g. bacon, pork loin); Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 52 Hot smoking at 65-90°C (e.g. eels, frankfurters, shellfish and salmon). 5.2 Definitions operator-defined limit means a measurable limit established by a risk management programme operator to manage the fitness for purpose of animal material or animal product regulatory limit means a measurable regulatory requirement that is critical to fitness for intended purpose of animal material or animal product 5.3 Regulatory Requirements Refer to Part 2 for the specific legal requirements under that apply to smoked products. 5.4 Procedures (for compliance) 5.4.1 Outcome of Smoking 5.4.1.1 The smoking process (together with any other hurdles applied to the product) must ensure the elimination or minimisation of the identified pathogens (e.g. Salmonella, E. coli O157:H7, Listeria monocytogenes, Staph. aureus and Clostridium perfringens) as determined through the application of HACCP principles, and must be sufficient to render the product microbiologically acceptable for its intended method of storage, intended purpose and shelf life. Where the smoking step is a means of preservation it would contribute to the achievement of this outcome. Where smoking is used for other purposes (e.g. sensory), the other process hurdles will be key to meeting the outcomes. 5.4.1.2 The operator must document any regulatory limits that are appropriate for the product. A regulatory limit is a measurable limit related to fitness for intended purpose, established by the Director-General. Regulatory limits may be expressed as a: i. product requirement, e.g. microbiological limit such as 0 cfu/gm Listeria monocytogenes in packaged cooked cured/salted meat; Further Processing Code of Practice Part 3: Good Operating Practice ii. July 2009 Amendment 1 Smoking Page 53 process parameters; iii. performance criteria, e.g. reduction in pathogen by 2 log. Regulatory limits are stated in the legislation e.g. the Food Standards Code, Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Currently there are few regulatory limits. 5.4.1.3 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and are not defined in legislation. Operator-defined limits may be categorised into the three types as described for regulatory limits. They may be taken from sources such as reputable COPs, peerreviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from their own trials and experiments. Examples of operator-defined limits could include microbiological criteria for a product that is not currently addressed within the Food Standards Code, or a required log reduction of a specified pathogen to be achieved by the process. In setting an operator-defined limit the operator should consider its intended use, the intended consumer and handling it is likely to receive after it has been released for sale. 5.4.2 Equipment Direct smoking is the traditional smoking process where the smoke is generated in the same chamber as the product is processed. Direct smoking exposes product to higher levels of PAHs (see section 5.4.3.2) and if the wood is not of a good standard when supplied and appropriately stored, opens up the possibility of cross contamination from wood dust and mould spores. Indirect smoking uses smoke generators which generate smoke in a separate chamber, the smoke may be cleaned by various methods before being fed into the smoking chamber. This generally results in reduced exposure to PAH and is a recommended technique. It is also recommended that operators design smoking and cooking chambers with the entry and exit on opposite walls, to allow for one way traffic of product only. This will reduce the chances of cross contamination from raw to processed product. Further Processing Code of Practice Part 3: Good Operating Practice 5.4.3 5.4.3.1 July 2009 Amendment 1 Smoking Page 54 Raw Materials Raw materials must comply with the requirements of the Food Standards Code. 5.4.3.2 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. 5.4.3.3 Smoke flavours are food additives that must comply with a specification listed under Standard 1.3.4 Identity and Purity of the Food Standards Code (e.g. the JECFA specification 2005 for smoke flavourings (wood smoke flavour, smoke condensate) has a definition, limits for benzo(a)pyrene and specified purity tests.) 5.4.3.4 Wood or other plant material used for the generation of smoke must: a. not contain toxic substances, either naturally occurring or through contamination with chemicals including paints, wood treatments or other impregnating materials; and b. be free from visible microbiological or fungal growth. There is currently no standard in the Food Standards Code for wood that is used to generate smoke for food processing. Wood product suppliers should follow good manufacturing practice. Operators should obtain supplier guarantees from their suppliers to confirm that untreated wood has been used to produce the wood shavings or sawdust. Wood shavings and sawdust should be sufficiently dry on delivery and should not be stored in large containers, heaps or silos to prevent spontaneous heating and growth of molds. Most commercial operations use sawdust which is easier to manage and gives greater smoke volume. The sawdust is often wet down to control burning and smoke density. Liquid smokes are increasingly being used instead of wood smoke as the process is more repeatable, the composition of the smoke is more constant, it is easier to apply and the carcinogenic compounds (e.g. PAH) have been minimised. Liquid smokes may be added directly to the product, or may be applied by dipping, spraying, or atomising the liquid smoke and injecting it into the smokehouse, vaporising the liquid on a hot surface, or by using smoke treated casings. Further Processing Code of Practice Part 3: Good Operating Practice 5.4.3.5 July 2009 Amendment 1 Smoking Page 55 The operator must consider the potential for the formation of chemical hazards such as polycyclic aromatic hydrocarbons (PAH) during the process, and where possible minimise product exposure to them. There is currently no standard requiring the measurement of PAHs in a smoked product. However, the operator should be aware of the conditions under which higher levels of PAHs are generated and wherever possible, manage those conditions to minimise their formation. The PAH level in the final product is dependant on a number of variables for example: the fuel used (hard vs. soft wood, traditionally only hardwoods have been acceptable for smoke generation); indirect smoking can produce lower PAH levels than direct smoking; filtering or cooling the smoke prior to use can result in lower PAH levels; product located closer to the heat source can have higher PAH levels; longer processing times can result in higher PAH levels; the temperature during processing can affect PAH levels; washing or water cooling the product after smoking can result in lower PAH levels; Keeping equipment clean and maintained can result in lower PAH levels. If steps are being taken to reduce PAH levels in a product the suitably skilled person needs to be mindful that conditions leading to a reduction in PAH may lead to increased levels of other chemical contaminants from the smoke or reduced microbiological safety. 5.4.4 5.4.4.1 Development and Validation of the Process The operator must have evidence to support and justify the selection of all operator-defined limits. 5.4.4.2 The operator must establish a process that will result in product that will meet any regulatory limits and operator-defined limits. Many interacting factors determine the preservative effect of smoking. The suitably skilled person Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 56 must be aware of these interactions and ensure that validation work is conducted under conditions that represent those that will be encountered during commercial operations. For example: the degree of diffusion of smoke into the interior of the product can vary. This is particularly important where products have been injected or reformed prior to smoking, with the possibility of pathogens being carried into the interior of the product where smoke may not penetrate. smoke extracts vary considerably in their ability to inhibit the growth of bacteria and testing must be conducted using the extract to be used in production. the time that smoke is applied to the product in relation to any drying or heating, and “skin” formation, must be considered particularly for seafood products. Where liquid smokes are blended throughout a product that is to be fermented, this may have an impact on the growth of the starter culture, by increasing the lag phase and thereby allowing pathogens to grow (Ellis, 2001). Where smoking is one of the hurdles used to preserve the product, the preservation factors described in other sections of this COP also apply, for example section 1, Heat treatments (including the development and validation, handling, cooling and operation of the process). Refer to other sections of the COP as relevant to the preservation factors to be applied. 5.4.4.3 Smoking processes that are relied upon to assist in the preservation of the product and are necessary for food safety must be developed and validated by a suitably skilled person, and revalidated by a suitably skilled person whenever there is a change to the process or product that would impact on its safety. It is important that the people who are responsible for the development and confirmation work have a good working knowledge of the factors that are critical to smoking. A suitably skilled person may be someone who has a good knowledge of: food microbiology and the growth parameters for the pathogens of concern; the interaction of the various hurdles, including smoking, in the preservation of the product; small changes in processing conditions (including the characteristics of the surface of the product) and its impact on the preservative effect of the smoke; the chemical hazards that may be generated during the smoking process; Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 57 any other hurdles used in the process and aspects of confirming the validity of those hurdles (e.g. heat treatment). It is the responsibility of the operator to ensure that people with the appropriate knowledge and skills are used. 5.4.4.4 Smoking processes that are relied upon to assist in the preservation of the product and are necessary for food safety must be developed, documented and validated considering all relevant factors. Factors to be considered will depend on the nature of the process and could include: micro-flora and microbial load of raw materials; potential microbiological growth; pre-smoking handling to minimise contamination and deterioration; type of casing or the surface of the product being smoked; method of smoke application (wood or extract); method of smoke generation; smoke density (the denser the smoke the greater the smoke uptake); air velocity (rapid air movement brings more smoke into contact with the product but can reduce smoke density, it can also lead to faster surface drying); step in the process that smoke is applied; smoking time; or concentration of liquid smoke; smoking temperature; relative humidity (high humidity or moisture content of the surface of the product favours smoke deposition but limits colour development); level of smoke deposition; potential for the development of chemical hazards; Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 58 any other preservation factors to be applied to the product and associated critical processing parameters (e.g. heat, salting, brining, drying, curing, refrigeration (refer to the relevant sections of this COP)); any product washing after smoking; product cooling; post-smoking product handling; storage conditions of finished product; any further preparation by the consumer. 5.4.4.5 5.4.5 5.4.5.1 The operator must keep records of the validation. Implementation The smoking process must be operated in accordance with the validated process and procedures. 5.4.5.2 Smoking must be carried out and/or supervised by appropriately trained personnel. Where the smoking step is a CCP, training expectations are heightened. The operator is to ensure that adequate training is provided and records of the training are kept. The training should cover the operation, control and monitoring of that step. 5.4.5.3 5.4.6 Records of the process must be retained for each production lot. Post-process handling Post-process handling refers to any processing after the smoking step and prior to the completion of packaging. Further Processing Code of Practice Part 3: Good Operating Practice 5.4.6.1 July 2009 Amendment 1 Smoking Page 59 Post-process handling must be performed so that recontamination is prevented and pathogen growth is minimised. 5.4.6.2 During post-process operations involving exposed product, access of persons from other incompatible areas must be restricted and an appropriate hygiene routine completed. For further information on pathogen management within the processing environment refer to the MAF document: Pathogen Management Plan Guidance Material (256 KB PDF), available in the Dairy section on the MAF website. 5.4.7 5.4.7.1 Packaging and Labelling Packaging must be of an appropriate composition for its intended use, storage times and conditions and method of product preparation by the consumer. Packaging which is used for a specific purpose such as modified atmosphere packaging, frozen storage or that is to remain in place during cooking must be of an appropriate composition for its intended use, storage conditions and method of product preparation by the consumer. 5.4.7.2 Systems must be put in place to check the packaging seal or closure integrity where necessary to ensure the safety of the product. This may include visual or physical testing e.g. complete seal, no cracking or wrinkling, maintenance of vacuum. 5.4.7.3 Any material used for sealing product such as metal clips, must be controlled in a manner that will ensure that it is not be a source of physical contamination to the product. 5.4.7.4 Product must be labelled in accordance with the requirements of the Food Standards Code, including any “Directions for Storage and Use” where specific methods of preparation are needed prior to consumption to ensure its safety. In some cases product that requires cooking prior to consumption is mistakenly being consumed as ready-to-eat product. Careful consideration should be given to the labelling to ensure that the need for cooking is clearly visible. Further Processing Code of Practice Part 3: Good Operating Practice 5.4.8 5.4.8.1 July 2009 Amendment 1 Smoking Page 60 Deviation from the Validated Process Action must be taken if there is a deviation from the critical parameters that results a final product that does not meet the validated process or product parameters, or would otherwise affect the safety of the product. 5.4.8.2 Any product from section 5.4.8.1 must be identified and segregated until its safety is assessed by a suitably skilled person. 5.4.8.3 A record of the assessment and corrective actions taken must be prepared by the suitably skilled person. The record should be appropriate to the nature of the deviation and should include: a. date and time of deviation; b. equipment identification, where appropriate; c. description of the nature and scope of the deviation; d. description of affected product, including code and quantity; e. corrective action taken, including restoration of control, product disposition and prevention of recurrence;; f. records of any tests undertaken; and g. the name and signature of the suitably skilled person. 5.4.9 5.4.9.1 Shelf-life The shelf-life of smoked products must be determined as part of the process development. 5.4.9.2 The processor must demonstrate adequate verification of the shelf-life expectancy. For further information on determining shelf life expectancy refer to the MAF document: A Guide to Calculating the Shelf Life of Foods. (460 KB PDF), available on the MAF website. Further Processing Code of Practice Part 3: Good Operating Practice 5.5 July 2009 Amendment 1 Smoking Page 61 References Codex. (2007). Proposed Draft Code of Practice for the Reduction of Contamination of Foods with Polycyclic Aromatic Hydrocarbons (PAH) from Smoking and Direct Drying Processes. CX/CF 07/1/16. Codex Committee on Contaminants in Foods. Rome: Codex Alimentarius Commission. Codex. (2005b). Proposed Draft Standard for Ready-to-Eat Smoked Fish. Appendix V. ALINORM 05/28/18. Codex Committee on Fish and Fishery Products. Rome: Codex Alimentarius Commission. Codex. (1979). Recommended International Code of Practice for Smoked Fish. CAC/RCP 25-1979. Rome: Codex Alimentarius Commission. Eklund, M. W., Peterson, M. E., Poysky, F. T., Paranjpye, R. N. and Pelroy, G. A. (2004). Control of Bacterial Pathogens During Processing of Cold-Smoked Salmon and Dried Salmon Strips. J of Food Protection, 67(2), 347-351. Ellis, D. F. (2001). Meat Smoking Technology In Meat Science and Applications. Eds Hui, Y. H., Wai-kit. Nip., Rogers, R. N., Young, O. A. Marcel Dekker, Inc. New York. Chpt 21. Faith, N. G., Yousef. A. E., Luchansky, J. B. (1992). Inhibition of Listeria monocytogenes by Liquid Smoke and Ioseugenol, a Phenolic Component Found in Smoke. J of Food Safety. 12, 303-314. Fletcher, G. C., Bremer, P. J., Summers, G. and Osborne, C. (2003). Guidelines for the Safe Preparation of Hot-Smoked Seafood in New Zealand. New Zealand Institute for Crop and Food Research Ltd. Gedela, S., Escoubas, J. R., Muriana, P. M. (2007). Effect of Inhibitory Liquid Smoke Fractions in Listeria monocytogenes During Long Term Storage of Frankfurters. J of Food Protection, 70(2), 386-391. Gilbert, S., Lake, R., Hudson, A. and Cressey, P. (2006). Risk Profile: Clostridium Botulinum in Ready-to-eat Smoked Seafood in Sealed Packaging. Institute of Environmental Science and Research Limited. Christchurch. N.Z. Jay, J. M. (2000). Modern Food Microbiology. Aspen Publishers Inc, Maryland. 6th Ed. Maga, J. A. (1998). Smoke in Food Processing. CRC Press Inc. US New South Wales Food Authority. Industry Guide to Developing a Food Safety Programme for Smoking Seafood. Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Smoking Page 62 Pearson, A. M. and Gillett, T. A. (1996). Processed Meats. Chapman and Hall, US. Taormina, P. J., Bartholomew, G. W. (2005). Validation of Bacon Processing Conditions to Verify Control of Clostridium perfringens and Staphyloccus aureus. J of Food Protection. 68(9). 1831-1839. USFDA. (2001). Processing Parameters Needed to Control Pathogens in Cold Smoked Fish. Varnam, A. H. and Sutherland, J. P. (1995). Meat and Meat Products. Technology, Chemistry and Microbiology. Chapman and Hall. UK. July 2009 Amendment 1 Acidification Further Processing Code of Practice Part 3: Good Operating Practice Page 63 6 Acidification 6.1 Purpose and Scope This section applies to the acidification of products to reduce the pH and achieve a preservation effect. Acids have an inhibitory and/or lethal effect on microorganisms depending on the levels used in the product. Typically organic acids such as acetic, malic or lactic acids are used, the type selected depending on a variety of factors such as flavour, physical form and effectiveness of achieving the required pH reduction. A pH of 4.6 is regarded as the limit for survival of Clostridia spp. and is the lower boundary of low acid foods for the purposes of commercial sterilisation. Other pathogens such as Escherichia coli O157:H7 and Listeria monocytogenes are able to survive in acid conditions more severe than pH 4. The following is a list of minimum pH values for growth for some foodborne pathogens: Bacillus cereus 4.3 Clostridium perfringens 5.0 E.coli O157:H7 4.4 Acid tolerant E.coli O157:H7 2.5 Listeria monocytogenes 4.1 Salmonella spp. 3.8 Staphylococcus aureus 4.0 6.2 Definitions equilibrium pH is the condition achieved when the solid and liquid parts of the product have the same pH. operator-defined limit means a measurable limit established by a risk management programme operator to manage the fitness for purpose of animal material or animal product Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Acidification Page 64 regulatory limit means a measurable regulatory requirement that is critical to fitness for intended purpose of animal material or animal product. 6.3 Regulatory Requirements Refer to Part 2 for the specific legal requirements that apply to acidified products. 6.4 Procedures (for compliance) 6.4.1 Outcome of Acidification 6.4.1.1 The operator must ensure the inhibition or inactivation of the identified vegetative pathogens (e.g. Salmonella, Listeria, Staph. aureus, E. coli 0157:H7) and of viable spores (e.g. Bacilli, Clostridia) as determined during the application of HACCP principles, and must be sufficient to render the product microbiologically acceptable for its intended method of storage, intended purpose and shelf life. 6.4.1.2 The operator must document any regulatory limits that are appropriate for the product. A regulatory limit is a measurable limit related to fitness for intended purpose, established by the Director-General. Regulatory limits may be expressed as a: i. product requirement, e.g. microbiological limit such as 0 cfu/gm Listeria monocytogenes in packaged cooked cured/salted meat; ii. process parameter; iii. performance criteria, e.g. reduction in pathogen by 2 log. Regulatory limits are stated in the legislation e.g. the Food Standards Code, Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. Currently there are few regulatory limits. 6.4.1.3 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Acidification Page 65 are not defined in legislation. Operator-defined limits may be categorised into the three types as described for regulatory limits. They may be taken from sources such as reputable COPs, peerreviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from their own trials and experiments. Examples of operator-defined limits could include the final pH of an acidified product, microbiological criteria for a product that is not currently addressed within the Food Standards Code, or a required log reduction of a specified pathogen to be achieved by the process. In setting an operator-defined limit the operator should consider its intended use, the intended consumer and handling it is likely to receive after it has been released for sale. 6.4.2 6.4.2.1 Raw Materials Raw materials must comply with the requirements of the Food Standards Code. 6.4.2.2 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. 6.4.3 6.4.3.1 Development and Validation of the Process The operator must have evidence to support and justify the selection of all operator-defined limits. 6.4.3.2 The operator must establish a process that will result in product that will meet any regulatory limits and operator-defined limits. 6.4.3.3 Acidification processes must be developed and validated by a suitably skilled person, and reconfirmed by a suitably skilled person whenever there is a change to the process or product that would impact on its safety. It is important that the people who are responsible for the development and confirmation work have a good working knowledge of the factors that are critical to acidification. A suitably skilled person may be someone who has a good knowledge of those critical factors and the importance of ensuring that the required pH is attained in the product and maintained for the duration of its shelf Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Acidification Page 66 life. The suitably skilled person will also need to have good knowledge of food microbiology and the growth parameters for the pathogens of concern, and the ability for acid adaptation by micro organisms and increased survival rates. It is the responsibility of the operator to ensure that people with the appropriate knowledge and skills are used. 6.4.3.4 Records of the validation must be retained. 6.4.3.5 Acidification processes must be developed and validated considering all relevant factors, such as: a. micro-flora and microbial load of raw materials; i. potential microbiological growth; b. product formulation, including ratio of solids to liquids in the case of brining, raw material pH variability, buffering capacity of the raw material; c. type and concentration of acidulant; d. the method of acidification (including addition and incorporation); e. time and conditions to achieve the equilibrium pH of all product components; f. equilibrium pH of final product; g. any other preservation factors to be applied to the product and associated critical processing parameters e.g. refrigeration, cooking; h. product handling; i. storage conditions of finished product; j. any further preparation by the consumer. Some common methods used to acidify products include: blanching the ingredients in acidified solutions; immersing blanched ingredients in acid solutions; direct batch acidification by adding a known amount of acid solution to a specified Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Acidification Page 67 amount of other ingredients; direct addition of a known amount of acid (generally in liquid form) to individual containers; addition of acid products to low-acid products in controlled proportions to conform to specific formulations. The type of evidence needed to validate the process will depend on the method of acidification. The key is to ensure that all parts of the equilibrated product meet the specified pH. 6.4.4 6.4.4.1 Implementation The process must be operated in accordance with the validated process and procedures. 6.4.4.2 The process must be verified at a frequency necessary to ensure that the established process and product parameters are consistently being met. 6.4.4.3 Where a pH meter is used to measure the acidity of a product: a. the measurement must be made using a properly calibrated instrument with an appropriate level of accuracy e.g. 0.05 pH units. b. the pH electrodes must be of a design appropriate to materials or products for which the pH is being measured. Measurements of acidity may be made by potentiometric methods (e.g. pH meter), titratable acidity or colorimetric methods (litmus or pH paper). If the finished equilibrium pH is above 4.0, the measurement should be made using a potentiometric method and any in-process measurements by titration or colorimetry related to that finished equilibrium pH. If the finished equilibrium pH is 4.0 or below, any suitable method of measuring acidity may be used for determining both finished product and any in-process pH levels. Care must be taken in calibration of the pH meter including the cleaning of electrodes, use of appropriate buffer solutions, sample temperature control and frequency of calibration etc. Further Processing Code of Practice Part 3: Good Operating Practice 6.4.4.4 July 2009 Amendment 1 Acidification Page 68 The acidification step must be carried out and/or supervised by appropriately trained personnel. Where a processing step is a CCP, training expectations are heightened. The operator is to ensure that adequate training is provided and records are kept. The training should cover the operation, control and monitoring of that step. Where the step does not meet the requirement for a CCP, the documented operating parameters should be regularly checked, verified and supervised by appropriately trained personnel. 6.4.4.5 6.4.5 6.4.5.1 Records of the process must be retained for each production lot. Additional Controls Acidified products which are not shelf stable must incorporate additional preservation controls such as refrigeration, thermal processing or reduced water activity. 6.4.6 6.4.6.1 Post-process Handling After acidification handling must be performed so that recontamination is prevented and the fitness for intended purpose of the product is maintained. 6.4.7 6.4.7.1 Packaging and Labelling Packaging must be of an appropriate composition for its intended use, storage times and conditions and method of product preparation by the consumer. Packaging which is used for a specific purpose such as modified atmosphere packaging, acidified product or that is to remain in place during cooking must be of an appropriate composition for its intended use, storage conditions and method of product preparation by the consumer. Further Processing Code of Practice Part 3: Good Operating Practice 6.4.7.2 July 2009 Amendment 1 Acidification Page 69 Where necessary to ensure the safety of the product, systems must be put in place to check the packaging seal or closure integrity. 6.4.7.3 Product must be labelled in accordance with the requirements of the Food Standards Code, including any “Directions for Storage and Use” where specific methods of preparation are needed prior to consumption to ensure its safety. 6.4.8 6.4.8.1 Storage The operator must take all reasonable steps to ensure that products are stored in a manner that will maintain their safety and wholesomeness for the duration of the shelf life. 6.4.9 6.4.9.1 Deviation from the Validated Process Action must be taken if there is a deviation from the critical parameters that results in a final product that does not meet the validated process or product parameters, or would otherwise affect the safety of the product. 6.4.9.2 Any product from section 6.4.9.1 must be identified and segregated until its safety is assessed by a suitably skilled person. 6.4.9.3 A record of the assessment and corrective actions taken must be prepared by the suitably skilled person. The record should be appropriate to the nature of the deviation and should include: a. date and time of deviation; b. equipment identification, where appropriate; c. description of the nature and scope of the deviation; d. description of affected product, including code and quantity; e. corrective action taken, including restoration of control, product disposition and prevention of recurrence; f. records of any tests undertaken; and Further Processing Code of Practice Part 3: Good Operating Practice July 2009 Amendment 1 Acidification Page 70 g. the name and signature of the suitably skilled person. 6.4.10 Shelf-life 6.4.10.1 The shelf-life must be determined as part of the process development. 6.4.10.2 The operator must demonstrate adequate verification of the shelf-life. For further information on determining shelf life expectancy refer to the MAF document: A Guide to Calculating the Shelf Life of Foods. (460 KB PDF), available on the MAF website. 6.5 References Canadian Meat Hygiene Manual of Procedures, Chapter 5 (Sampling and Testing) and Chapter 15.4 (Establishment: Hygienic Processing Requirements). Guide to Inspections of Acidified Food Manufacturers, FDA (1 p 10 of 22). Jay, J. M. (2000). Modern Food Microbiology. Aspen Publishers, Inc. Maryland. 6th Ed. Microbial Pathogen Data Sheets, MAF website, retrieved 2007. Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 71 7 High Pressure Processing 7.1 Purpose and Scope This section contains the requirements for high pressure processing (HPP) where it is applied for the purpose of producing a safe product, by reducing the identified pathogens to acceptable levels. It does not apply to processes that are intended to achieve a technological effect only such as the shucking of shellfish or meat extraction from crustaceans. HPP (also known as high hydrostatic pressure (HHP) and ultra high pressure processing (UHP)) involves subjecting liquid or solid foods, with or without packaging, to pressures of between 100 and 1000 MPa to inactivate pathogens and extend a product’s shelf life. Almost always, products that are subject to HPP only (without the addition of sterilising temperatures or other means of preservation) are not shelf stable at room temperature and so must be stored under refrigeration. The resistance of microorganisms to pressure is highly variable and is not usually correlated to heat resistance. It depends primarily on the type of organism and the food matrix involved. For most forms of vegetative bacteria, significant reductions (usually higher than 4 log units) can be achieved when pressures of 400-600 MPa are used. However, under these conditions, significant reductions in the load of spores are not achieved. (Rendueles at al, 2010). 7.2 Definitions HPP or HP process means high pressure process operator-defined limit means a measurable limit established by a risk management programme operator to manage the fitness for purpose of animal material or animal product regulatory limit means a measurable regulatory requirement that is critical to fitness for intended purpose of animal material or animal product suitably skilled person means a person who in the opinion of the operator is skilled in a particular activity or task through training, experience or qualifications. Further Processing Code of Practice Part 3: Good Operating Practice 7.3 August 2011 Amendment 1 High Pressure Processing Page 72 Regulatory Requirements Refer to Part 2 for the specific legal requirements that apply to HP processed products. 7.4 Procedures (for compliance) 7.4.1 7.4.1.1 Outcome of High Pressure Processing Where the aim of the process is to achieve a safe product (rather than a technological effect only) the operator must ensure the inactivation of identified vegetative pathogens (e.g. S. aureus, Salmonella spp, E. coli O157:H7, Listeria monocytogenes) and the inhibition of viable spores (e.g. Bacilli, Clostridia) as determined during the application of HACCP principles, and the process must be sufficient to render the product microbiologically safe for its intended shelf life and storage conditions. 7.4.1.2 The operator must document any regulatory limits that are appropriate for the product. A regulatory limit is a measurable limit related to fitness for intended purpose, established by the Director-General. Regulatory limits may be expressed as a: i. product requirement, e.g. microbiological limit such as 0 cfu/25 gm Listeria monocytogenes in Bivalve molluscs that have undergone processing other than depuration 2 ; ii. process parameter, e.g. compression pressure; iii. performance criteria, e.g. reduction in pathogen by 2 log. Regulatory limits are stated in the legislation, for example in the Food Standards Code (External website) or the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004. (434 KB PDF). Currently there are few regulatory limits. 2 Where HPP is used as a physical measure to shuck shellfish and the product is sold raw, the Lm criteria do not apply. In this case HPP is not considered to be a process step other than depuration. August 2011 Amendment 1 High Pressure Processing Further Processing Code of Practice Part 3: Good Operating Practice 7.4.1.3 Page 73 The operator must establish and document operator-defined limits for the product. Operator-defined limits are additional measurable limits that are established by the operator and are not defined in legislation. Operator-defined limits may be categorised into the three types as described for regulatory limits. They may be taken from sources such as reputable COPs, peerreviewed scientific information, predictive models, scientific information from a person or organisation known to be competent, or developed from their own trials and experiments. Examples of operator-defined limits could include microbiological criteria for a product that is not currently addressed within the Food Standards Code, a required log reduction of a specified pathogen to be achieved by the HP process, or HP processing parameters. In setting an operatordefined limit the operator should consider the products intended use, intended consumer and handling it is likely to receive after it has been released for sale. 7.4.2 7.4.2.1 Equipment HPP equipment must be fitted with control systems that are capable of generating permanent process records and identifying process deviations. As a minimum HHP systems should be fitted with transducers for the measurement of pressure, time, and temperature of the process. The following is an example of the transducers, their precision and accuracy, and their location in the vessel (FDA, 2000). Measuring Transducer Accuracy/Precision Location in Pressure Vessel Pressure Gauge (Electronic) +/- 1/2% / 3.4 MPa Anywhere in HPP system Pressure Gauge (Dial Display) +/- 1% / 6.8 MPa Anywhere in HPP system Temperature (Thermocouple) +/- 1/2% / 0.5 °C Vessel cold point or its equivalent Time (Recorder) +/- 1% / one second (not applicable) Further Processing Code of Practice Part 3: Good Operating Practice 7.4.2.2 August 2011 Amendment 1 High Pressure Processing Page 74 All equipment used for critical measurements must meet clause 28 of the Animal Products (Specifications for Products Intended for Human Consumption) Notice 2004 which relates to calibration. [See Part 2] For example, pressure and temperature transducers used in controlling a process should be calibrated periodically against traceable instruments. The frequency of calibration will be a function of the number of pressure cycles and should be determined by testing. 7.4.2.3 7.4.3 7.4.3.1 Equipment must be installed and commissioned by suitably skilled people. Raw Materials Raw materials must comply with the requirements of the Food Standards Code. 7.4.3.2 Raw materials must be of an appropriate microbiological status. The operator should have knowledge of the microbiological status of raw materials. This information may be obtained from certificates of analysis from suppliers, from the National Microbiological Database for some meat types, by testing the raw materials, or using information gained from a previous processing step. The capability of the process, intended use of the product and any further processing and/or preparation prior to consumption should be considered when determining the microbiological criteria for raw materials. 7.4.3.3 Raw materials must be protected from contamination, handled hygienically and stored in a manner that will minimise deterioration to ensure that they will remain fit for their intended purpose and method of processing. Further Processing Code of Practice Part 3: Good Operating Practice 7.4.4 7.4.4.1 August 2011 Amendment 1 High Pressure Processing Page 75 Development and Validation of the High Pressure Process The operator must have evidence to support and justify the selection of all operator-defined limits. 7.4.4.2 The operator must establish an HP process that will result in product that will meet any regulatory limits and/or operator-defined limits. 7.4.4.3 Product trials must be conducted to validate process parameters derived from any of the following: h. calculations; i. data from similar processes; j. reference material; k. computer modelling programmes. The complexity of the validation will depend on the nature of the operation, the identified hazards and the availability of established process parameters or criteria. For example: for a simple or standard process, validation may only require trials to demonstrate that the process is operated strictly in accordance with process parameters and/or criteria obtained from a reputable agency or research institute, or published in a scientific journal. These may include previously validated parameters such as pressure, time, any temperatures and critical product formulation constraints for a novel or more complex process, the operator will need to establish that their own process parameters and/or criteria are capable of producing safe product, and then provide evidence from trials which demonstrate achievement of those parameters challenge trials (or trials where the ingoing level of the target pathogen(s) is known prior to processing) would be expected for complex processes and recommended for simple processes, so that the level of kill achieved by the process is demonstrated Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 76 The number of trials needed will be very variable. For example, in the first scenario above, a single trial only may be required to confirm that the parameters can be met and that safe product will be produced, which could happen during the commissioning. For the second scenario, more trials would be expected and for the third scenario, one trial might be sufficient if the target pathogens were inoculated at levels above which they would ever be expected to be found in the product. Where challenge trials depend on natural levels of contamination a greater number of trials in which the pathogen is confirmed to be present at the start of the trial would be expected. The levels of the pathogen should be quantified before and after each trial, and should closely approximate the worst case levels that might be found in the raw material. 7.4.4.4 HP processes must be developed and validated by a suitably skilled person, and revalidated by a suitably skilled person whenever there is a change to the process or product that would impact on its safety. It is important that people who are responsible for validation have a good working knowledge of the factors that are critical to HPP. The suitably skilled person will also need to have good knowledge of food microbiology and the various factors that impact on pressure resistance. It is the responsibility of the operator to ensure that people with the appropriate knowledge and skills are used. 7.4.4.5 The following information must be addressed when developing an HP process. This list is not exhaustive. Suitably skilled persons must use their experience to ensure that all relevant factors are addressed. a. name of the person that developed the process; b. date of development; c. product name, code, type and formulation; d. preparation, filling and closure of product; e. identification of microbiological hazards and spoilage organisms associated with the raw materials and their microbial loadings; f. test conditions used to design the process, including the data acquisition system; Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 77 g. any other preservation hurdles to be applied to the product; h. critical process parameters such as: i. minimum initial product temperature; ii. initial temperature of pressure transmitting fluid and process vessel; iii. time to bring vessel to pressure; iv. pressure of vessel during process; v. process time at pressure; vi. in the case of pulsed processes: number of pulses, pause time-interval between pulses, pause pressure, product temperature and holding time for each pulse (Balasubramaniam, 2004); vii. process temperature (if temperature is specified as an integral part of the preservation process); viii. decompression time; i. log reduction of target pathogen; j. packaging and storage conditions of finished product. It is important that the process is developed using the product as formulated because ingredients such as acids, salt and fat content etc will affect, for example, pH and water activity, which are critical process factors in the inactivation of pathogens in foods treated by HPP (FDA, 2000). The monitoring and control of pH and water activity in formulated products should be included in the HACCP plan. Composition also appears to have a major affect on the degree of heating during processing. Rasanayagam et al (2003) showed that for the same pressure time profile, soy oil increased to 60°C whereas water increased only to 40°C from the same initial temperature. It is generally recognised that the compression process will increase the temperature of the water by approximately 3°C or more per 100 MPa and fats and oils could increase by 8-9°C per 100 MPa. If no heat is gained or lost during the hold phase, the food cools to its original temperature on decompression. If considering using microbiological surrogates in favour of pathogenic strains of vegetative bacteria during trials it should be noted that even different strains of the same pathogen may have significantly different responses to pressure. As such the process should be designed to protect Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 78 against the most resistant strain of pathogen likely to be encountered. It should also be noted that pressure alone cannot be relied upon for the inactivation of spore forming pathogens. Viruses may also need to be considered. Further research is needed in this area. Pressure resistance of pathogens does not necessarily align with temperature resistance and the suitably skilled person will also need to consider the possible impact of “tailing”. This is where a proportion of the population is not inactivated and may be brought about by the existence of subpopulations of cells that are particularly resistant to pressure due to better defence and repair systems (Rendueles at al, 2010). Hartmann et al (2003) looked at the influence of heat and mass transfer on uniformity of high pressure induced inactivation. They found significant differences of more than one log cycle in the residual surviving cell concentration depending on the package material parameters, position and arrangement of the packages in the vessel, indicating that these factors can have a major effect on inactivation during processing. The initial temperature in the vessel before introducing foods should be specified and the product should be at an initial homogeneous temperature with no cold spots. Where temperature is specified in the process, it will need to be monitored. This may be limited to recording the initial temperature of the product and the processing medium where the process is performing consistently. Where temperature is not part of the specified process the operator should still record the test temperatures as part of the validation work and ensure that trials are conducted which cover the temperature ranges likely to be encountered during processing. Worst case temperatures which deliver the lowest level of pathogen inactivation will need to be covered (note that this may not necessarily be the lowest temperature). This should include product and processing medium temperatures. Long come-up times will also affect inactivation kinetics of microorganisms therefore, consistency and awareness of these times are important in the process development. Further Processing Code of Practice Part 3: Good Operating Practice 7.4.4.6 August 2011 Amendment 1 High Pressure Processing Page 79 Records of the validation must be retained for the production life of the product and for a further four years. 7.4.5 Implementation 7.4.5.1 The process must be operated in accordance with the validated process and procedures. 7.4.5.2 Product must be processed promptly. The maximum time from package filling to HP processing must be specified, taking into consideration conditions which may permit microbial growth and toxin production. Ideally, the time should not exceed 2 hours unless evidence of an acceptable alternative is available. 7.4.5.3 Where the appearance of HPP product is such that it could mistakenly bypass the HP process, procedures and/or systems must be put in place to prevent this from occurring. 7.4.5.4 The process must be verified at a frequency necessary to ensure that the established process and product parameters are consistently being met. 7.4.5.5 The process must be carried out and/or supervised by appropriately trained personnel. Where a processing step is a CCP, training expectations are heightened. The operator is to ensure that adequate training is provided and records of the training are kept. The training should cover the operation, control and monitoring of that step. 7.4.5.6 Permanent records of the process must be retained for each production lot. The recording system used should contain instrumentation that will signal process deviations outside the validated limits. Records should include the: Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 80 product formulation product initial temperature time to bring vessel to pressure pressure of vessel during process process time at pressure process temperature: The temperature within the vessel should be measured at a standard point so that any deviations are consistently recorded. The probe should be located to measure the worst case temperature in the vessel, i.e. the temperature that will be the least effective in activating the targeted pathogen. decompression time checks on package integrity (FDA, 2000) 7.4.5.7 The operator must ensure that no changes are made to any critical factor, including product formulation or HP process unless validated by a suitably skilled person, prior to operation. 7.4.6 7.4.6.1 Post-process Handling Where product is exposed to the processing environment after the HPP step, product handling must be performed so that recontamination is prevented and pathogen growth is minimised. 7.4.7 7.4.7.1 Packaging and Labelling Packaging must be of an appropriate composition for its intended use, storage times and conditions, and method of product preparation by the consumer. Due to the compression and re-expansion of the food during the HP process, the packaging needs to be capable of withstanding up to a 15% reduction in volume without losing seal integrity or barrier properties (USFDA, 2000). Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 81 Packaging which is subject to the pressures of HPP or that is to remain in place during further preparation by the consumer such as cooking must be of an appropriate composition and in the case of cooking, be designed for use at high temperatures. 7.4.7.2 Where necessary to ensure the safety of the product, systems must be put in place to check the packaging seal or closure integrity. 7.4.7.3 Product must be labelled in accordance with the requirements of the Food Standards Code, including any “Directions for Storage and Use” where specific methods of preparation are needed prior to consumption to ensure its safety. 7.4.8 7.4.8.1 Deviation from the Validated Process Corrective action must be taken if there is a deviation from the validated process or product parameters that would affect the safety of the final product, where the product is intended to be traded. 7.4.8.2 Any product from section 7.4.8.1 must be identified and segregated until its safety is assessed by a suitably skilled person. 7.4.8.3 A record of the assessment and corrective actions taken must be prepared by the suitably skilled person. The record should be appropriate to the nature of the deviation and should include: a. date and time of deviation; b. equipment identification, where appropriate; c. description of the deviation; d. description of affected product, including code and quantity; e. corrective action taken, including restoration of control, product disposition and prevention of recurrence; f. records of any tests undertaken; and g. the name and signature of the suitably skilled person. Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 82 For example, a complete loss of process pressure could require reprocessing. A 10% loss of process pressure, for a known time, could be corrected by an additional holding time at the specified pressure, provided the pressure could be regained immediately after the deviation (FDA, 2000). Where a corrective action such as extending the pressurised holding time is undertaken the operator would need to have evidence that the new process is capable of delivering a safe product. If the deviation may have affected the microbiological loading of the product prior to applying the HP process (e.g. the maximum holding time or temperature was exceeded) the operator would need to have evidence that the HP process applied was capable of reducing the increased microbiological loading to acceptable levels. 7.4.9 7.4.9.1 Shelf-life The shelf-life must be determined as part of the process development. During shelf life testing, consideration should be given to the possibility of the product containing sub lethally injured microorganisms which may be rendered inactive by HPP but could recover during storage. This may be investigated by extending the time products are tested for beyond their stated shelf life. 7.4.9.2 The operator must demonstrate adequate verification of the shelf-life. For further information on determining shelf life expectancy refer to the MAF document: A Guide to Calculating the Shelf Life of Foods. (460 KB PDF). 7.5 References Balasubramaniam, V. M., Ting, E. Y., Stewart, C. M., Robbins, J. A. (2004) Recommended laboratory practices for conducting high-pressure microbial inactivation experiments. Food Science and Emerging Technologies. 5, 299-306. Hartmann, C., Delgado, A., Szymczyk, J. (2003) Convective and diffusive transport effects in a high pressure induced inactivation process of packed food. Journal of Food Engineering. 59(1), 33-44. Rasanayagam, V., Balasubramaniam, V, M., Ting, E., Sizer, C, E., et al. (2003) Compression heating of selected fatty food materials during high-pressure processing. Journal of Food Science 68(1), 254–259. Further Processing Code of Practice Part 3: Good Operating Practice August 2011 Amendment 1 High Pressure Processing Page 83 Rendueles, E., Omer, M.K., Alvseike, O., Alonso-Calleja, C., Capita, R.,Prieto, M. (2010). Microbiological Food Safety Assessment of High Hydrostatic Pressure Processing: A Review. Food Science and Technology, 44(5), 1251-1260. Smelt, J. P. (1998). Recent Advances in the Microbiology of High Pressure Processing. Trends in Food Science and Technology, 9, 152-158. USFDA (2000). Kinetics of Microbial Inactivation for Alternative Food Processing Technologies. http://www.fda.gov/Food/ScienceResearch/ResearchAreas/SafePracticesforFoodProcesses/ ucm101456.htm