April 2
advertisement

Molecular Spectroscopy
Chapter 13
An introduction to Ultraviolet/Visible Absorption Spectroscopy
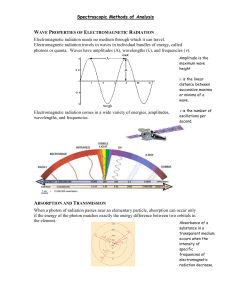
In this chapter, absorption by molecules, rather than atoms, is considered. Absorption
in the ultraviolet and visible regions occurs due to electronic transitions from the
ground state to excited state. Broad band spectra are obtained since molecules have
vibrational and rotational energy levels associated with electronic energy levels. The
signal is either absorbance or percent transmittance of the analyte solution where:
P
Po
A = log Po/P or P = P0*10-A
%T = (P/Po)*100%
The absorbance or %T can be related to concentration and are thus the basis of
analyte determination. Factors affecting absorbance, linearity of calibration plots, as
well as instruments used in the UV-Vis spectroscopy will be detailed in this chapter.
Important Terms and Symbols in UV-Vis Spectroscopy
The technique of UV-Vis spectroscopy is one of the oldest spectroscopic techniques
where terminology and symbols have changed with time. A list of old and new
symbols and terms are summarized in the table below:
Term and Symbol
(recommended)
Definition
Radiant power (P, Po)
Absorbance (A)
Energy of radiation
Log (Po/P)
Transmittance (T)
Path length of radiation (b)
Absorptivity (a)
Molar absorptivity (ε)
P/Po
-A/bc (c in g/L)
A/bc (c in mol/L)
Beer’s Law
Old Terms and Symbols
(avoid use)
Radiation intensity (I, Io)
Optical Density (D), or
Extinction (E)
Transmission (T)
l or d
Extinction coefficient (k)
Molar extinction coefficient
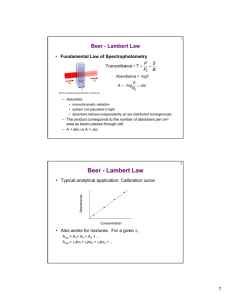
The relation between absorbance and concentration is best described by Beer’s law.
This law can be easily obtained by simple argument as follows:
It is absolutely correct to assume that the absorbance of a solution of analyte, when
light of a suitable wavelength passes through, is directly proportional to analyte
concentration © as well as the path length (b) of the light beam through solution:
A α bc
The two terms will be equal when multiplying the second term by a constant (called
molar absorptivity, ε). This will give the relation:
A = εbc
This relation is called Beer’s law. Sometimes this relation is referred to as Lambert
law or Beer’s-Lambert law, but we will use the most popular name of the relation
which is Beer’s law.
The Beer’s law equation suggests a linear relation between absorbance and
concentration with no intercept. However, there are some factors that affect the
linearity of the relation which will be detailed shortly.
Applications of Beer’s Law to Mixtures
In case of coexistence of two or more absorbing species in solution, all will absorb if
incident beam contains suitable wavelengths. The overall absorbance of the solution
will be the sum of all absorbances of the different components:
Atotal = A1 + A2 + A3 + …..
Atotal = ε1bc1 + ε2bc2 + ε3bc3 + ….
However, it becomes too difficult to perform calculations involving absorbance from
more than two absorbing species.
Limitations to Beer’s Law
Three major reasons for limitations of Beer’s law, which tender the relation between
absorbance and concentration deviate from linearity, can be identified:
1. Real Limitations
a. Beer’s law is good for dilute analyte solutions only. High concentrations
(>0.01M) will cause a negative error since as the distance between molecules
become smaller the charge distribution will be affected which alter the
molecules ability to absorb a specific wavelength. The same phenomenon is
also observed for solutions with high electrolyte concentration, even at low
analyte concentration. The molar absorptivity is altered due to electrostatic
interactions.
b. In the derivation of Beer’s law we have introduced a constant (ε). However, ε
is dependent on the refractive index and the refractive index is a function of
concentration. Therefore, ε will be concentration dependent. However, the
refractive index changes very slightly for dilute solutions and thus we can
practically assume that ε is constant.
c. In rare cases, the molar absorptivity changes widely with concentration, even
at dilute solutions. Therefore, Beer’s law is never a linear relation for such
compounds, like methylene blue.
2. Chemical Deviations
This factor is an important one which largely affects linearity in Beer’s law. It
originates when an analyte dissociates, associates, or reacts in the solvent. For
example, an acid base indicator when dissolved in water will partially dissociate
according to its acid dissociation constant:
HIn = H+ + InIt can be easily appreciated that the amount of HIn present in solution is less than that
originally dissolved where:
CHIn = [HIn] + [In-]
Assume an analytical concentration of 2x10-5 M indicator (ka = 1.42x10-5) was used,
we may write:
1.42x10-5 = x2/(2x10-5 – x)
Solving the quadratic equation gives:
X = 1.12x10-5 M which means:
[In-] = 1.12x10-5 M
[HIn] = 2x10-5 – 1.12x10-5 = 0.88x10-5 M
Therefore, the absorbance measured will be the sum of that for HIn and In-. If a 1.00
cm cell was used and the ε for both HIn and In- were 7.12x103 and 9.61x102 Lmol1
cm-1 at 570 nm, respectively, the absorbance of the solution can be calculated:
A = AHIn + AIn
A = 7.12x103 * 1.00* 0.88x10-5 + 9.61x102 * 1.00 *1.12x10-5 = 0.073
However, if no dissociation takes place we may have:
A = AHIn
A = 7.12x103 * 1.00 * 2x10-5 = 0.142
If the two results are compared we can calculate the % decrease in anticipated signal
as:
% decrease in signal = {(0.142 – 0.073)/0.142}x100% = 49%
An example of association equilibria is the association of chromate in acidic solution
to form the dichromate according to the equation below:
2 CrO42- + 2 H+ = Cr2O72- + H2O
The absorbance of the chromate ions will change according to the mentioned
equilibrium and will thus be non linearly related to concentration.
3. Instrumental Deviations
a. Beer’s law is good for monochromatic light only since ε is wavelength
dependent. It is enough to assume a dichromatic beam passing through a
sample to appreciate the need for a monochromatic light. Assume that the
radiant power of incident radiation is Po and Po’ while transmitted power is P
and P’. The absorbance of solution can be written as:
A = log (Po + Po’)/(P + P’)
P = Po10-εbc, substituting in the above equation:
A = log (Po + Po’)/(Po10-εbc Po’10-ε’bc)
Assume ε = ε’ = ε
A = log (Po + Po’)/(Po + Po’) 10-εbc
A = εbc
However, since ε’ # ε, since ε is wavelength dependent, then A # εbc
A
λ
Therefore, the linearity between absorbance and concentration breaks down if
incident radiation was polychromatic. In most cases with UV-Vis spectroscopy,
the effect small changes in wavelengths is insignificant since since ε differs only
slightly; especially at the wavelength maximum.
b. Stray Radiation
Stray radiation resulting from scattering or various reflections in the instrument will
reach the detector without passing through the sample. The problem can be severe in
cases of high absorbance or when the wavelengths of stray radiation is in such a range
where the detector is highly sensitive as well as at wavelengths extremes of an
instrument. The absorbance recorded can be represented by the relation:
A = log (Po + Ps)/(P + Ps)
Where; Ps is the radiant power of stray radiation.