Intro to spectrochemical methods
advertisement

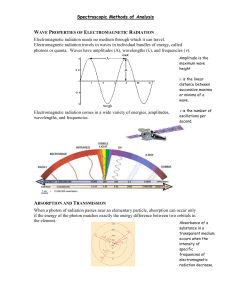
Chapter 24 Introduction to Spectrochemical Methods • Measurements based on electromagnetic radiation are widely used in analytical chemistry. • The interactions of radiation and matter are the subject of spectroscopy. • Spectroscopic analytical methods are based on measuring the amount of radiation produced or absorbed by molecular or atomic species of interest. UV-VISIBLE (UV-Vis)spectroscopy Classification • Classify spectroscopic methods according to region of the electromagnetic spectrum involved: γ–ray, Xray, ultraviolet (UV), visible, infrared (IR), microwave, and radio frequency (RF). • Spectrochemical methods provide the most widely used tools for the elucidation of molecular structure as well as the quantitative and qualitative determination of both inorganic and organic compounds. • RELEVANT EXAMPLES FOR PHARMACY FOLLOW UV-VISIBLE spectroscopy cont… INFRARED (IR) spectroscopy ATOMIC EMISSION (AES) spectroscopy INFRARED (IR) spectroscopy cont… ATOMIC ABSORPTION (AAS) spectroscopy FLUORESCENCE (FS) spectroscopy Properties of Electromagnetic Radiation • Electromagnetic radiation is energy • Electromagnetic radiation can be described as a wave (wavelength, frequency, velocity, and amplitude) • Electromagnetic radiation can be treated as discrete packets of energy or particles called photons or quanta. • These dual views of radiation as particles and waves are not mutually exclusive but complementary. Nuclear Magnetic Resonance NMR The Speed of Light • In vacuum, c= 2.99792 x 108 m s-1 • In air speed is about 0.03% less. • Thus, for a vacuum, or for air, the velocity of light to three significant figures is: c =νλ = 3.00 x 108m s-1 = 3.00 x 1010 cm s-1 • In all other mediums (eg glass or liquids) speed of light is much less than c because of interaction between the electromagnetic field and electrons in the atoms or molecules of the medium. • In these cases, the frequency of the radiation is constant, - passes therefore the wavelength must decrease as the light from a vacuum to a medium containing matter Light in air travelling through glass. The particle Nature of Light: Photons Energy of photon is related to its wavelength, or frequency, or wavenumber by E = hν = hc/λ = hcν where, h is Planck’s constant (6.63 x 10-34 J s). The wavenumber ν and frequency ν, in contrast to the wavelength λ, are directly proportional to the photon energy E. The radiant power of a beam of radiation is directly proportional to the number of photons per second. The Electromagnetic Spectrum • The electromagnetic spectrum covers a very wide range of energies (frequencies) and wavelengths. • From 1019 Hz (γ-rays) to 103 Hz (radio waves). • Different quantum changes (energy level changes) in atoms/molecules are caused by radiation with different frequencies. Spectroscopic Measurements Interactions of radiation with mater is used to get information about a sample. Sample is stimulated by applying energy (heat, electrical energy, light, particles, or a chemical reaction.) The analyte in the sample is predominately in its lowestenergy or ground state. The stimulus causes some analyte species to undergo a transition to a higher-energy or excited state. We obtain information about the analyte either by measuring the electromagnetic radiation emitted as it returns to the ground state or by measuring the amount of electromagnetic radiation absorbed during excitation. …continued… • Emission spectroscopy usually refers to methods in which the stimulus is heat or electrical energy. • chemiluminescence spectroscopy refers to excitation of the analyte by a chemical reaction. • Measurement of the radiant power emitted as the analyte returns to the ground state can give information about its identity and concentration. • The results of such a measurement are often expressed graphically by a spectrum, a plot of the emitted radiation as a function of frequency or wavelength. …continued… • When sample is stimulated by irradiation some of the incident radiation is absorbed and promotes some analyte species to an excited state. – In absorption spectroscopy, the amount of light absorbed as a function of wavelength is measured, which can give qualitative and quantitative information about the sample. – In photoluminescence spectroscopy the emission of photons at different wavelength is after absorption. These are called fluorescence and phosphorescence spectroscopy. ABSORPTION EMISSION The Absorption LAW • Beer-Lambert law or Beer’s law, • Relates amount of radiation absorption to concentration of absorbing species and pathlength over which absorption occurs. • As light crosses a medium containing an absorbing analyte, decreases in intensity occur as the analyte becomes excited. • For a given analyte concentration, the longer the pathlength, the more absorbers are in the path and therefore greater the absorption. • Also for a given pathlength of light, the higher the concentration of absorbers, the stronger the attenuation. Beer’s Law The attenuation (decrease in intensity) of a parallel beam of monochromatic radiation as it passes through an absorbing solution of thickness b cm and concentration c moles per liter. Because of interactions between the photons and absorbing particles, the radiant power of the beam decreases from P0 to P. The transmittance T of the solution is the fraction of incident radiation transmitted by the solution. Transmittance is often expressed as a percentage and called the percent transmittance. T = P / P0 The absorbance A of a solution is related to the transmittance in a logarithmic manner. A= -logT= log(P0/P) Measuring Transmittance and Absorbance Ordinarily, transmittance and absorbance, cannot be measured as shown because the solution to be studied must be held in some sort of container (cell or cuvette). Reflection and scattering losses can occur at the cell walls. These losses can be substantial. Light can also be scattered in all directions from the surface of large molecules or particles, such as dust, in the solvent, and this can also cause further attenuation of the beam as it passes through the solution. …continued… To compensate for these effects, the power of the beam transmitted through a cell containing the analyte solution is compared with one that traverses an identical cell containing only the solvent or a reagent blank. An experimental absorbance that closely approximates the true absorbance for the solution is thus obtained; that is A = log P0 / P ≈ log Psolvent / Psolution Beer’s Law According to Beer’s law, absorbance A is directly proportional to the concentration of the absorbing species c and the pathlength b of the absorbing medium A = log P0 / P = abc Here, a is a proportionality constant called the absorptivity. Because absorbance is a unitless quantity, the absorptivity must have units that cancel the units of b and c. If, for example, c has the units of grams per liter (g L-1) and b has the units of centimeters (cm), absorptivity has the units of liters per gram centimeter (L g-1 cm-1). …continued… Applying Beer’s Law to Mixture When we express the concentration in moles per liter and b in centimeters, the proportionality constant, called the molar absorptivity, is given the special symbol ε. Thus, A = εbc where, ε has the units of liters per mole centimeter (L mol-1 cm-1). Beer’s law also applies to solutions containing more than one kind of absorbing substance. Provided that there are no interactions among the various species, the total absorbance for a multicomponent system is the sum of the individual absorbances. In other words, Atotal = A1 + A2 + … An = ε1bc1 + ε2bc2 + … + εnbcn where the subscripts refer to absorbing componets 1, 2, …, n. Limits to Beer’s Law There are few exception to the linear relationship between absorbance and pathlength at a fixed concentration. We frequently observe deviations from the direct proportionality between absorbance and concentration where b is a constant. Some of these deviations, called real deviations, are fundamental and represent real limitations to the law. Others occur as a consequence of the manner in which the absorbance measurements are made or as a result of chemical changes associated with concentration changes. These deviations are called instrumental deviations and chemical deviation respectively. Chemical Deviations Real Limitations to Beer’s Law Beer’s law describes the absorption behavior of dilute solutions only and is a limiting law. At concentrations exceeding about 0.01 M, the average distances between ions or molecules are diminished to the point where each particle affects the charge distribution, and thus the extent of absorption of its neighbors. The occurrence of this phenomenon causes deviations from the linear relationship between absorbance and concentration. When ions are in close proximity, the molar absorptivity of the analyte can be altered because of electrostatic interactions, which can lead to departures from Beer’s law. Instrumental Deviations The need for monochromatic radiation and the absence of stray radiation are practical factors that limit the applicability of Beer’s law. Beer’s law strictly applies only when measurements are made with monochromatic source radiation. If the band selected corresponds to a region in which the absorptivity of the analyte is essentially constant, departures from Beer’s law will be minimal. Many molecular bands in the UV/visible region fit this description. To avoid deviation, it is advisable to select a wavelength band near the wavelength of maximum absorption where the analyte absorptivity changes little with wavelength. Deviations from Beer’s law appear when the absorbing species undergoes association, dissociation, or reaction with the solvent to give products that absorb differently from the analyte. The extent of such departures can be predicted from the molar absorptivities of the absorbing species and the equilibrium constants for the equilibria involved. Unfortunately, we are usually unaware that such processes are affecting the analyte, so compensation is often impossible. Typical equilibria that give rise to this effect include monomer dimer equilibria, metal complexation equilibria where more than one complex is present, acid/base equilibria, and solvent-analyte association equilibria. …continued… Stray radiation, commonly called stray light, is defined as radiation from the instrument that is outside the nominal wavelength band chosen for the determination. This stray radiation is often the result of scattering and reflection off the surfaces of gratings, lenses or mirrors, filters, and windows. When measurements are made in the presence of stray light, the observed absorbance is given by A` = log P 0 + Ps P + Ps where Ps is the radiant power of the stray light. …continued… Stray light always causes the apparent absorbance to be lower than the true absorbance. The deviations due to stray light are most significant at high absorbance values. Because stray radiation levels can be as high as 0.5% in modern instruments, absorbance levels above 2.0 are rarely measured unless special precautions are taken or special instruments with extremely low stray light levels are used. Another deviation is caused by mismatched cells. If the cells holding the analyte and blank solutions are not of equal pathlength and equivalent in optical characteristics, and intercept will occur in the calibration curve. This error can be avoided either by using matched cells or by using a linear regression procedure to calculate both the slope and intercept of the calibration curve. Absorption spectra An absorption spectrum is a plot of absorbance versus wavelength. Absorbance could also be plotted against wavenumber or frequency. Most modern scanning instruments can produce such an absorption spectrum directly. Older instruments often display transmittance and produce plots of %T versus wavelength. Occasionally, plots with logA as the ordinate are used. A plot of molar absorptivity ε as a function of wavelength is independent of concentration. Atomic Absorption When a beam of polychromatic uv/visible radiation passes through a medium containing atoms, only a few frequencies are attenuated by absorption. The spectrum consists of a number of very narrow absorption lines. Transition between two different orbitals are termed electronic transitions. Atomic absorption is measured at a single wavelength using a very narrow monochromatic source. Molecular Absorption Three types of energy changes occur when molecules are excited by ultraviolet, visible,and infrared radiation. For UV/visible radiation, when we excite a molecule, an electron residing in a lowenergy molecular or atomic orbital is promoted to a higher-energy orbital. The change in energy levels is called a transition. We can only get such a transition when the energy hν of the photon is exactly the same as the energy difference between the two orbital energies. The transition of an electron between different energy levels is called an electronic transition, and the absorption process is called electronic absorption. …continued… Molecules exhibit two additional types of radiationinduced transitions: vibrational transitions and rotational transitions. The vibrational energy of a molecule is associated with the bonds that hold the molecule together. The vibrational energy of a molecule is quantized and can only assume certain discrete levels. Transitions between vibrational levels are vibrational transitions. The overall energy E associated with a molecule in a given state can be written as E = Eelectronic + Evibrational + Erotaitonal where Eelectronic is the electronic energy of the molecule, Evibrational is its vibrational energy, and Erotaitonal is its rotational energy. Energy-Level Diagrams for Molecule A partial energy-level diagram depicting typical processes that occur when a polyatomic molecule absorbs infrared, visible and ultraviolet radiation is shown. The energies E1 and E2 of the first two of the several electronically excited states of a molecule are shown relative to the energy of its ground state associated with each electronic state are indicated by the lighter horizontal lines labeled 1, 2, 3, and 4. The lowest vibrational levels are labeled 0. Infrared Absorption Infrared radiation is generally not of sufficient energy to cause electronic transitions but can induce transitions in the vibrational and rotational states associated with the ground electronic state of the molecule. For absorption to occur, the analyte must be irradiated with frequencies corresponding exactly to the energies indicated by the lengths of the four arrows (wavelengths λ1 –λ4 as shown). Ultraviolet and Visible Absorption The center arrows in the energy diagram suggest that the molecules under consideration absorb visible radiation of five wavelengths (λ1 to λ5), thereby promoting electrons to the five vibrational levels of the excited electronic level E1. Ultraviolet photons that are more energetic are required to produce the absorption to level E2 shown by the five arrows on the right (wavelengths λ1 to λ5).