L22
advertisement

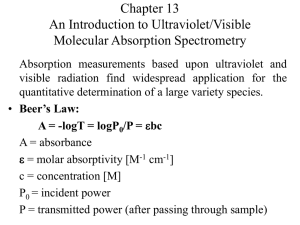
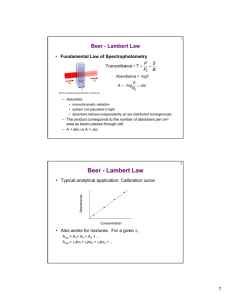
UV-Vis Absorption Spectroscopy Lecture 22 1 Measurement of Transmittance and Absorbance: The power of the beam transmitted by the analyte solution is usually compared with the power of the beam transmitted by an identical cell containing only solvent. An experimental transmittance and absorbance are then obtained with the equations. P0 and P refers to the power of radiation after it has passed through the solvent and the analyte. 2 Beer’s law and mixtures • Each analyte present in the solution absorbs light! • The magnitude of the absorption depends on its e • A total = A1+A2+…+An • A total = e1bc1+e2bc2+…+enbcn • If e1 = e2 = en then simultaneous determination is impossible • Need to measure A at nl’s (get 2n e’s) to solve for the concentration of species in the mixture 3 4 5 Limitations to Beer’s Law • Real limitations • Chemical deviations • Instrumental deviations 6 1. a. 7 Real Limitations Beer’s law is good for dilute analyte solutions only. High concentrations (>0.01M) will cause a negative error since as the distance between molecules become smaller the charge distribution will be affected which alter the molecules ability to absorb a specific wavelength. The same phenomenon is also observed for solutions with high electrolyte concentration, even at low analyte concentration. The molar absorptivity is altered due to electrostatic interactions. b. In the derivation of Beer’s law we have introduced a constant (e). However, e is dependent on the refractive index and the refractive index is a function of concentration. Therefore, e will be concentration dependent. However, the refractive index changes very slightly for dilute solutions and thus we can practically assume that e is constant. c. In rare cases, the molar absorptivity changes widely with concentration, even at dilute solutions. Therefore, Beer’s law is never a linear relation for such compounds, like methylene blue. 8 2. Chemical Deviations This factor is an important one which largely affects linearity in Beer’s law. It originates when an analyte dissociates, associates, or reacts in the solvent, or one of matrix constituents. For example, an acid base indicator when dissolved in water will partially dissociate according to its acid dissociation constant: 9 10 11 12 13 Chemical deviations from Beer’s law for unbuffered solutions of the indicator Hln. Note that there are positive deviations at 430 nm and negative deviations at 570 nm. At 430 nm, the absorbance is primarily due to the ionized In- form of the indicator and is proportional to the fraction ionized, which varies nonlinearly with the total indicator concentration. At 570 nm, the absorbance is due principally to the undissociated acid Hln, which increases nonlinearly with the total concentration . 14 Calculated Absorbance Data for Various Indicator Concentrations 15 16 3. a. 17 Instrumental Deviations Beer’s law is good for monochromatic light only since e is wavelength dependent. It is enough to assume a dichromatic beam passing through a sample to appreciate the need for a monochromatic light. Assume that the radiant power of incident radiation is Po and Po’ while transmitted power is P and P’. The absorbance of solution can be written as: 18 19 The effect of polychromatic radiation on Beer’s law. In the spectrum at the top, the absorptivity of the analyte is nearly constant over Band A from the source. Note in the Beer’s law plot at the bottom that using Band A gives a linear relationship. In the spectrum, Band B corresponds to a region where the absorptivity shows substantial changes. In the lower plot, note the dramatic deviation from Beer’s law that results. 20 21 Therefore, the linearity between absorbance and concentration breaks down if incident radiation was polychromatic. In most cases with UVVis spectroscopy, the effect small changes in wavelengths is insignificant since e differs only slightly; especially at the wavelength maximum. 22 b. Stray Radiation Stray radiation resulting from scattering or various reflections in the instrument will reach the detector without passing through the sample. The problem can be severe in cases of high absorbance or when the wavelengths of stray radiation is in such a range where the detector is highly sensitive as well as at wavelengths extremes of an instrument. The absorbance recorded can be represented by the relation: A = log (Po + Ps)/(P + Ps) Where; Ps is the radiant power of stray radiation. 23 24