Beer's Law Class Notes

Spectroscopic Methods of Analysis
W AVE P ROPERTIES OF E LECTROMAGNETIC R ADIATION
Electromagnetic radiation needs no medium through which it can travel.
Electromagnetic radiation travels in waves in individual bundles of energy, called photons or quanta. Waves have amplitudes (A), wavelengths (
λ
), and frequencies (
ν
).
Electromagnetic radiation comes in a wide variety of energies, amplitudes, wavelengths, and frequencies.
Amplitude is the maximum wave height
λ is the linear distance between successive maxima or minima of a wave.
ν
is the number of oscillations per second.
A BSORPTION AND T RANSMISSION
When a photon of radiation passes near an elementary particle, absorption can occur only if the energy of the photon matches exactly the energy difference between two orbitals in the element.
Absorbance of a substance in a transparent medium occurs when the intensity of specific frequencies of electromagnetic radiation decrease.
Spectroscopic Methods of Analysis
Some substances can absorb radiation in specific wavelengths of the visible range of the electromagnetic spectrum and this is practical for obtaining quantitative chemical information through the use of a spectrophotometer or a colorimeter.
I I o
Colorimeter
For absorbing species, the intensity of visible light before passing through the chemical sample is higher than the intensity of visible light that has passed through the sample. This ratio of incident radiation (I) to outputted radiation (I o
) is called transmittance (T).
T = I o
/I
Transmittance (T) is a measure of the quantity of light that passes through a medium.
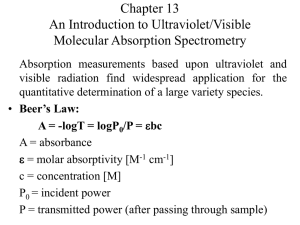
Absorbance, A, is related to transmission by the equation
A = – log
10
T
B EER ’ S L AW AND B EER ’ S L AW P LOT
If a sample absorbs visible light, the amount of absorbing particles is related to the absorbance by
A
α
moles
Moles in solution are measured by molar concentration, c, therefore,
A
α
c
A plot of absorbance versus concentration is called a Beer’s Law Plot and the equation describing this relationship is called the Beer—Lambert Law or Beer’s Law, expressed as
A =
ε bc where
ε
is a proportionality constant and the slope of a Beer’s Law Plot, called the molar absorptivity. b is the pathlength of the radiation through the absorbing medium in cm.
A Beer’s Law Plot and Beer’s Law is used in quantitative analysis to determine the concentration of an unknown sample from absorbance data.
Spectroscopic Methods of Analysis
Sample Problem
Since absorbance is a unitless value, calculate the units of the molar absorptivity constant,
ε
.
L IMITATIONS OF THE B EER -L AMBERT LAW
The linearity of the Beer-Lambert law is limited by chemical and instrumental factors.
Causes of nonlinearity include:
•
•
•
•
• deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity scattering of light due to particulates in the sample fluoresecence or phosphorescence of the sample changes in refractive index at high analyte concentration
Analyte is the substance being measured in an analytical procedure. shifts in chemical equilibria as a function of concentration
• non-monochromatic radiation, deviations can be minimized by using a relatively flat part of the absorption spectrum such as the maximum of an absorption band
• stray light