Chapter 9: Phase Diagrams - Course Notes
advertisement
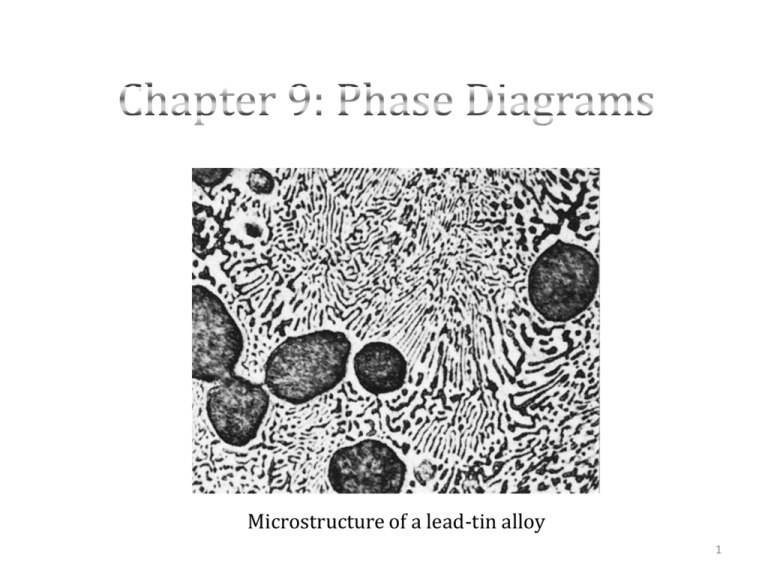
Microstructure of a lead-tin alloy 1 Example: Building Structures Burj Dubai under construction 2007; completed 2010 Example: Aerospace Industry F/A22 Raptor Single-Crystal Titanium panels Example: Aerospace Industry Pratt and Whitney F119 Engine Why study Phase Diagrams? • Provide valuable information about: – Melting – Casting – Microstructure – Crystallization – Temperature – Pressure – Composition – Phase transformations Phase Diagrams • Component: a chemical constituent of an alloy, which may be used to specify its composition – Example: In a copper-zinc brass, the components are Cu and Zn • Solid solution (Chapter 4): – Consists of atoms of at least two different types – Solute atoms occupy either substitutional or interstitial positions – Crystal structure of solvent is maintained Solubility Limit • Maximum concentration of solute atoms that may dissolve in the solvent, at a specific temperature, to form a solid solution • Adding solute past the solubility limit yields another solid solution (different crystal structure) or compound that has a different composition Phases • Phase: a homogeneous portion of a system that has uniform physical and chemical characteristics • The following are considered phases: – Solid solution – Liquid solution – Gaseous solution • If more than one phase is present in a system, each will have its own distinct properties • Phases exist over a range of compositions, temperatures and pressures • Example: Water and Ice two phases (physically dissimilar, but chemically the same) 8 Stability of Phase • Phase stability depends on temperature, pressure and composition, 9 Phases For each of the following situations how many phases are present? 1. Ice cubes floating in a glass of water. 2. Water at room temperature containing 20 wt% sugar. 3. A bottle of 7-Up. 10 Components vs. Phases • Components: – The elements (or compounds) that are mixed to make the alloy • Al and Cu • MgO and Al2O3 • styrene and butadiene – The composition is the concentration of each component present in an alloy • Phase: Al-Cu alloy Phase α – Physically and/or chemically distinct regions in the material Phase β Aluminum atom Copper atom 11 Sugar- Water Demo • Initially, sugar is added to water and there is a sugar- water solution or syrup • Solution becomes more concentrated with sugar until the solubility limit is reached (saturated) • System cannot dissolve any more sugar (at the specified temperature) and further additions settle to the bottom of the container • System now contains two phases; syrup and solid (undissolved) sugar crystals Solid Solubility • We define solubility as the maximum equilibrium concentration of a solute in a host material. ALS: What is the solubility of sugar in water at 20oC? Answer: 65 wt% sugar Solid Solubility • We define solubility as the maximum equilibrium concentration of a solute in a host material Previous Answer: 65 wt% sugar ALS: If you have 1 L (1000 g) of water, how much sugar can you dissolve in it? a) 650 g of sugar b) 2000 g of sugar c) 1350 g of sugar Solid Solubility • We define solubility as the maximum equilibrium concentration of a solute in a host material Previous Answer: 65 wt% sugar ALS: If you have 1 L (1000 g) of water, how much sugar can you dissolve in it? Answer: The solution is 65% (about 2/3) sugar and 1/3 water. The weight of the sugar is twice that of the water, so 2000 g is the answer. Solubility Limits If you leave a saturated water/sugar solution open to the air and the water starts to evaporate, what will happen? Answer: At some point there won’t be enough water to hold all of the sugar. The solubility for sugar will be exceeded, and some of it will come out of the solution in crystal form (i.e. precipitate) to compensate. Phase Diagrams 17 • • • • One-Component (Or Unary) Phase Diagrams 3 externally controllable parameters that affect phase structure: – Temperature – Pressure (usually scaled logarithmically) – Composition Unary phase diagrams only deal with pressure-temperature graphs composition remains constant Along the phase boundaries, the phases on either side are in equilibrium Triple point: all three states are in equilibrium (at 273.16 K at a pressure of 6.04x10-3 atm in the example) (H20) Each of the phases will exist in equilibrium conditions over the corresponding temperature 18 Binary Phase Diagrams 19 Binary Phase Diagrams • Temperature and composition are variable parameters • Binary alloys – contain two components • Pressure held constant normally ≈ 1 atm • Maps that represent the relationship between temperatures, compositions and quantities of phases at equilibrium • Accurately predict phase transformations and resulting microstructures Binary Isomorphous Systems • Easiest binary phase diagram to understand – Example: copper-nickel system • Temperature is plotted along the vertical axis • Composition of the alloy is plotted along the horizontal axis – Bottom- weight % – Top – atom % – Composition ranges from 0 wt% Ni (100 wt% Cu) on the left of the horizontal axis to 100 wt% Ni (0 wt% Cu) on the right of the horizontal axis Binary Isomorphous Systems • Three phase regions or fields: – Alpha(α) • Substitutional solid solution consisting of Cu and Ni and both have an FCC crystal structure – Liquid (L) • Homogeneous liquid solution composed of both copper and nickel – Two phase α + L • The copper-nickel system is termed isomorphous due to the complete liquid and solid solubility of the two components Phase Boundary Interpretation of Phase Diagrams • For a binary system of known composition and temperature that is at equilibrium, you can identify: 1. Phases present 2. Compositions of phases 3. Percentages or fractions of phases • Phases Present: – Locate the temperature-composition point on the diagram and one can identify the phase(s) within the phase field that are/ is present Interpretation of Phase Diagrams • Phase compositions: – One phase present: • composition of phase is the same as the overall composition of the alloy – Two phases present: • A Tie line is constructed across two-phase region at a specified temperature of the alloy • Intersections of tie lines and phase boundaries on both sides are noted • The compositions corresponding to these points are read from the x-axis. Interpretation of Phase Diagrams • Determining Phase Amounts: – One phase present: • Alloy is composed entirely of that phase; phase fraction is 1.0 or percentage is 100% – Two phases present: • Use the lever rule; tie line is constructed across the two-phase region at a specified temperature of the alloy • Overall alloy composition is located on the tie line • Fraction of one phase is determined by taking the length of the tie line from the overall alloy composition to the phase boundary for the other phase, and dividing by the total lie line length • The fraction of the other phase is determined in the same manner • Segment lengths can be determined either by direct measurement from the phase diagram using ruler or by subtracting compositions from the composition axis Alternate Approach - Phase diagrams • Liquid mass fraction: C C0 S WL R S C CL • α-Phase mass fraction: C0 CL R W R S C CL Developing Microstructures – Isomorphous Alloys • Equilibrium cooling: – Cooling occurs very slowly Mechanical Properties of Isomorphous Alloys • Solute Strengthening TS for pure Ni %EL for pure Ni %EL for pure Cu TS for pure Cu Binary Eutectic Systems One common type of alloy system is called the eutectic system. Binary eutectic systems occur when 2 elements completely dissolve into each other in the liquid state, but have only limited solubility in the solid state. More than one solid phase exists in these systems. There are multiphase regions between single-phase regions. 29 Which phases are present depends, as before, on the temperature and the composition of the material. Binary Eutectic Systems • Consider Cu-Ag alloys: The copper-silver alloy system is binary eutectic. − How many phases are there in the system? 3 (L, , b) − What are the two solid phases? : mostly Cu b: mostly Ag − Where is the eutectic point? eutectic temperature: TE eutectic composition: CE 30 ALS: Pb-Sn Eutectic System The lead-tin system is also binary eutectic • Consider an alloy which contains 40 wt% Sn at 150oC. • What phases are present? What are their compositions? • Answer: There are 2 phases: a and b. Ca = 11 wt% Sn Cb = 99 wt% Sn 31 ALS: Pb-Sn Eutectic System • How much of each phase is present? • Answer: From the lever rule: 59 W 67% 88 Wb 1 W 33% 32 Microstructures in Eutectic Systems • The result is... – … a single phase polycrystal with grains. Adapted from Fig. 9.9, 33 Callister 6e. L Temperature oC • Consider a eutectic system (e.g. Pb-Sn) with an initial concentration (Co) less than 6 wt% Sn. 400 300 L 200 b 100 0% Co 10% %Sn 20% 30% Microstructures in Eutectic Systems • Consider a eutectic system with an initial concentration (Co) between 2 and 18.3 wt% Sn. • The result is... – … a 2-phase polycrystal with... • … and β grains. • … a fine grained structure. Adapted from Fig. 9.10, 34 Callister 6e. L: Cowt%Sn T(°C) 400 L 300 200 TE 100 L+ L : Cowt%Sn b +b Pb-Sn system 0 10 20 30 Co Co, wt% 2 (sol. limit at Troom) 18.3 (sol. limit at TE) Sn Microstructures in Eutectic Systems • When the initial concentration (Co) equals the eutectic composition (CE) the result is... – … alternating layers of and β crystals Pb-Sn system Adapted from Fig. 9.12, Callister 6e. (Fig. 9.12 from Metals Handbook, Vol. 9, 9th ed., Metallography and Microstructures, American Society for Metals, Materials Park, OH, 1985.) Adapted from Fig. 9.11, 35 Callister 6e. Microstructures in Eutectic Systems • Co between 18.3 and 61.9 wt% Sn results in... – … a combination of crystals and eutectic regions L L 300 Pb-Sn system L+ 2 00 TE R R 100 0 0 b S 40 Co Callister 6e. C L = 61.9 wt% Sn W = + b 20 18.3 Just above TE: C = 18.3 wt% Sn L+ b S 60 61.9 Adapted from Fig. 9.14, 36 L L: Cowt%Sn T(°C) 80 primary eutectic eutectic b 100 97.8 wt% Sn S = 50 wt% R+S WL = (1-W) = 50 wt% Microstructures in Eutectic Systems • Co between 18.3 and 61.9 wt% Sn results in... – … a combination of crystals and eutectic regions Just below TE: Pb-Sn system C = 18.3 wt%Sn C b = 97.8 wt%Sn W = S = 73 wt% R+S Wb = 27 wt% Adapted from Fig. 9.14, 37 Callister 6e. Eutectoid and Peritectic Reactions • Eutectoid reaction: a reaction wherein upon cooling, one solid phase transforms isothermally and reversibly into two new solid phases that are intimately mixed cooling heating • Peritectic reaction: a reaction wherein upon cooling, a solid and a liquid phases transform isothermally and reversibly to a solid phase having a different composition L Copper-zinc phase diagram cooling heating 38 The Iron-Carbon System 39 The Iron-Iron Carbide (Fe-Fe3C) Phase Diagram • When heated, pure iron experiences two changes in its crystal structure before it reaches its melting temperature: – At room temperature, exists as ferrite (α iron) and has a BCC structure – Ferrite experiences a polymorphic transformation to FCC austenite (γ iron) at 912°C – Experiences a second transformation to BCC phase known as δ ferrite at 1394°C, which finally melts at 1538°C The Iron-Iron Carbide (Fe-Fe3C) Phase Diagram • Cementite, (Iron Carbide - Fe3C). It is 25at% C or 6.7 wt% C. The Iron-Iron Carbide (Fe-Fe3C) Phase Diagram Eutectic reaction for the iron-iron carbide system: L cooling heating Euctectic Point Fe3C Eutectoid Point Eutectoid reaction for the iron-iron carbide system: (0.76wt %C) cooling heating (0.022wt %C) Fe3C(6.7wt %C) Development of Microstructure in Iron-Carbon Alloys • The microstructure depends on: – Concentration of carbon – Heat treatment • Pearlite: a two-phase microstucture results from the transformation of austenite and consists of alternating layers ( or lamellae) of -ferrite and cementite Pearlite 43 Hypoeutectoid Alloys • Hypoeutectoid alloy : (less than eutectoid) between 0.022 and 0.76wt% C • Proeutectoid ferrite: that formed above the eutectoid temperature 44 Hypereutectoid Alloys • Hypereutectoid alloys: containing between 0.76 and 2.14 wt% C • Proeutectoid cementite: that which forms before the eutectoid reaction cementite composition remains constant as temperature changes 45 Non-Equilibrium Cooling • In most situations, the cooling rates are impractically slow and unnecessary • Nonequilibrium effects of practical importance: – Phase changes or transformations at temperatures other than those predicted in phase diagrams – The existence of non-equilibrium phases that do not appear on the phase diagram 46 Chapter 9 Review What did you learn? • • • • • • 47 Chapter 9 Practice Problems Practice Problems 1. Salt is often spread on roads during the winter in order to depress the freezing point of water. A binary phase diagram for water and salt (NaCl) is shown below. Liquid (Brine) Field “Z” Practice Problems a) What are the two phases that exist in equilibrium in the field “Z” region. b) At -10°C determine the overall composition for which equal amounts of these phases exist in equilibrium. c) What are the compositions of each phase under these conditions? d) What is the lowest temperature at which adding salt can prevent water from freezing completely? Practice Problems Answer: a)Liquid (Brine) + Ice b)8 wt% NaCl c)Ice – 0.5 wt% NaCl Salt + Brine – 16 wt% NaCl d) -21°C Practice Problems 2. Using the Fe-Fe3C phase diagram, compute the weight fractions of proeutectoid ferrite and pearlite, respectively, that form in an iron-carbon alloy containing 0.25 wt% C if the alloy is slowly cooled to a temperature just below the eutectoid temperature. Practice Problems a) Wα = 0.31, b) Wα = 0.69, c) Wα = 0.56, d) Wα = 0.44, Wp = 0.69 Wp = 0.31 Wp = 0.44 Wp = 0.56 Practice Problems Answer: 0.76 0.25 W 0.69 0.76 0.022 0.25 0.022 Wp 0.31 0.76 0.022 Therefore, the answer is b) 54 Practice Problems c) Before eutectic reaction : 61.9 30 W 0.7317 61.9 18.3 After eutectic reaction : 97.8 30 W 0.8528 97.8 18.3 formed during eutectic reaction : W 0.8528 0.7317 0.1211or 12.11% 55