Orbital Diagrams & Electron Configurations for Atoms and Ions
advertisement

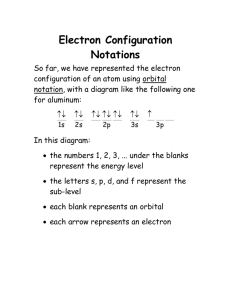
Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital diagrams represent the arrangement of electrons in orbitals. • boxes or lines represent each orbital • arrows within boxes represent the electrons • max two per box • opposite direction (represents opposite spin) Section 3.5 e.g., Hydrogen (Z=1) e.g., Helium (Z=2) 1s The aufbau principle: • The atom is “built up” by progressively adding electrons. • Electrons will fill the lowest available energy levels first, before filling higher levels. 1s Within a principal energy level, s<p<d<f Example: 3s fills before 3p But… the (n+1)s orbitals always fill before the nd orbitals. Example: 5s fills before 4d Practice! Orbital filling order for elements beyond Period 2 … ...corresponds to atom’s location in periodic table! Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing orbital diagrams 1s 1. Fill orbitals in order of increasing energy. 2s 2p 2. Orbitals of the same energy level must be filled before moving onto the next energy level. 3. Place electrons singly into orbitals within a sublevel before Hund’s rule: To achieve the any pairing occurs. lowest possible energy, electrons will spread out singly before pairing up within an orbital. 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number of electrons • cation (positive): REMOVE appropriate number of electrons Example: Oxide ion, O2- Before beginning, ask yourself: a) How many Draw orbital diagrams for: a) Sulfur (Z=16) 1s e- Practice! in an oxygen atom? b) How many e- do I add/remove? c) What’s the new total number of e-? 2s 2p b) Iron (Z=26) c) Aluminum (Z=13) Tip: Use lines instead of boxes to represent the orbitals. An electron configuration is a shorthand notation that provides the same information as the orbital diagram. Practice! Draw orbital diagrams for: b) Iron (Z=26) d) Gold (Z=79) B boron 10.81 a) Sulfur (Z=16) c) Aluminum (Z=13) 5 Tip: Use lines instead of boxes to represent the orbitals. Guidelines for writing electron configurations Practice! 1. Write out subshells that have electrons in them. 2. Use superscripts to indicate how many electrons are in each subshell. Write electron configurations for: a) Sulfur (Z=16) b) Iron (Z=26) c) Aluminum (Z=13) d) Gold (Z=79) Electron configurations can be abbreviated: Two exceptions to the pattern: Cr and Cu Gold: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d9 Possible explanation: Filled and half-filled sub-shells have lower energy than unfilled ones more stable = [Xe] 6s2 4f14 5d9 Nearest preceding noble gas Practice! Write abbreviated electron configurations for the atoms you just completed Electron configurations can explain periodic properties. Chromium: [Ar] 4s 3d Learning Checkpoint You can use the position on the periodic table to write out the electron configuration for an element. Elements in a group: • same valence electron configuration • common properties: chemical, physical Electron configurations of ions Na: 1s2 2s2 2p6 3s1 Na+: Mg: 1s2 2s2 2p6 3s2 1s2 2s2 2p6 Al: 1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 Why do atoms form ions? All of these ions are ISOELECTRONIC with Neon. Weird quirk: Even though the s orbitals fill first, they lose first when forming ions Zn: Zn2+: O: 1s2 2s2 2p4 1s2 2s2 2p6 • • • • 3d10 “Transition Metals” d-block elements examples? more orbitals = more possibilities [Xe] 6s2 4f14 5d106p2 Lose 2e- 3d Pb2+: Summary • Orbital diagrams use boxes or lines to visually represent the orbitals of atoms. Arrows are used to represent electrons. 1s2 2s2 2p6 MO ORBITALS MO PROBLEMS Pb: …also gives up more easily [Xe] • Electron configurations convey the same information, but in a more condensed form. 1s2 2s2 2p5 Multivalent metals can have more than one ionic charge. [Ar] [Ar] 4s0 3d10 4s easier to fill… 4s2 F: F- : 6s2 4f14 5d106p0 Lose 4e- Pb4+: [Xe] 6s0 4f14 5d106p0