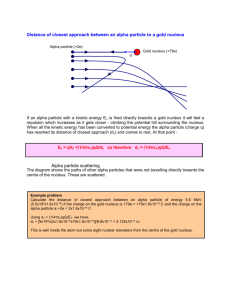
nucleus The calcium-40 atom has the mass number A = 40 and the
advertisement

The calcium-40 (Ca-40) nucleus The calcium-40 atom has the mass number A = 40 and the atomic number Z = 20, so its standard model has 20 protons, 20 neutrons and 20 electrons. In the alpha-beta model, a mass number of 40 and an atomic number of 20 means that the nucleus contains 40 aetrons and 20 zetrons configured as 10 alpha particles. Since the argon-36 nucleus already contains nine alpha particles, adding one more alpha particle to it makes calcium-40. The atomic mass of calcium-40 is 39.9625 amu. Multiplying this by the factor 1,824 bmu/amu converts it to 72,892 bmu. Since each electron in the atom has a mass of 1 bmu, subtracting 20 bmu from this to remove the mass of the 20 electrons leaves an calcium-40 nuclear mass of 72,872 bmu. The argon-36 nucleus has a mass of 65,586 bmu. Adding the mass of an alpha particle to this increases the mass by 7,298 bmu to 72,884 bmu. If the alpha particle attaches to the argon-36 nucleus in either of the locations shown in figure below, it sits atop of the alphas in argon-36 such that three of its nucleons make contact with three nucleons in the argon-36. This creates three single bonds. Since each single bond reduces the total mass of the system by 4 bmu, attaching the alpha particle to the argon-36 nucleus with three single bonds reduces the 72,884 bmu total mass of the two components by 12 bmu. This makes the final calcium-40 model mass 72,872 bmu. This is equal to the measured value. It appears that the calcium-40 nucleus could have formed whenever an alpha particle collided with an argon36 nucleus in the right orientation. However, it more likely formed when a neon-20 nucleus attached to another neon-20 nucleus with nine single bonds. The neon-20 nucleus sits on a unused face of the other neon-20 nucleus to make calcium-40. The mass of the neon nucleus is 36,454 bmu and the mass of the other neon-20 nucleus is also 36,454 bmu, so their combined masses equal 72,908 bmu. The nine single bonds attaching the two nuclei together reduce their total mass by 36 bmu, leaving a calcium-40 mass of 72,872 bmu, the same as above.