Empirical Formulas
advertisement
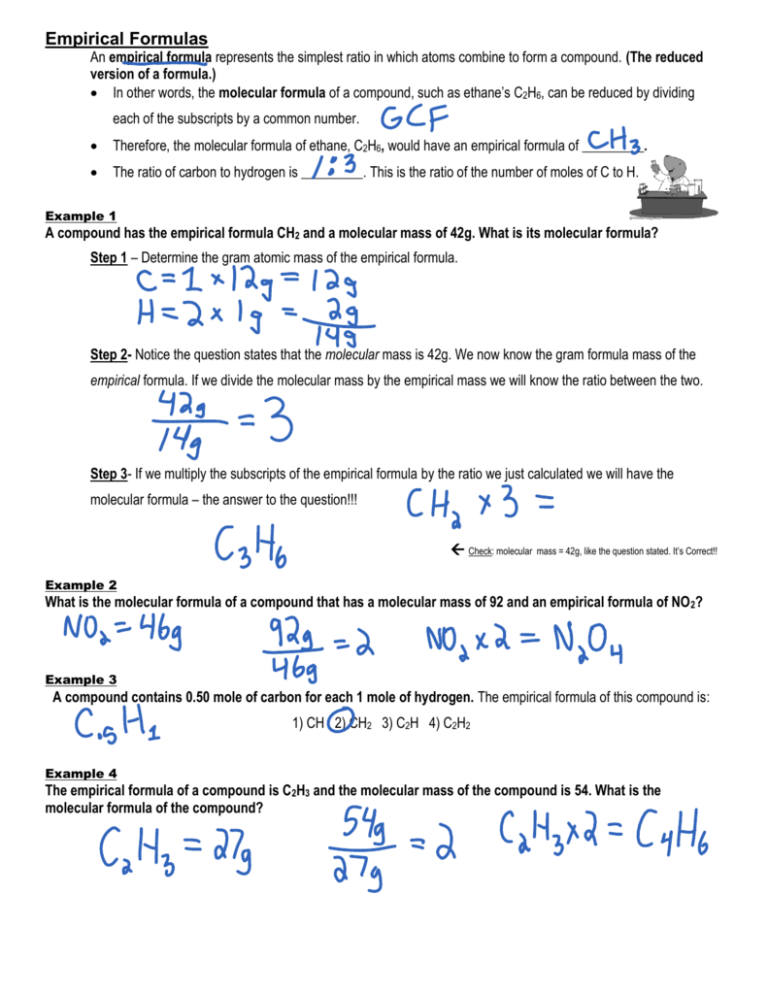
Empirical Formulas An empirical formula represents the simplest ratio in which atoms combine to form a compound. (The reduced version of a formula.) In other words, the molecular formula of a compound, such as ethane’s C2H6, can be reduced by dividing each of the subscripts by a common number. Therefore, the molecular formula of ethane, C2H6, would have an empirical formula of _________. The ratio of carbon to hydrogen is _________. This is the ratio of the number of moles of C to H. Example 1 A compound has the empirical formula CH2 and a molecular mass of 42g. What is its molecular formula? Step 1 – Determine the gram atomic mass of the empirical formula. Step 2- Notice the question states that the molecular mass is 42g. We now know the gram formula mass of the empirical formula. If we divide the molecular mass by the empirical mass we will know the ratio between the two. Step 3- If we multiply the subscripts of the empirical formula by the ratio we just calculated we will have the molecular formula – the answer to the question!!! Check: molecular mass = 42g, like the question stated. It’s Correct!! Example 2 What is the molecular formula of a compound that has a molecular mass of 92 and an empirical formula of NO 2? Example 3 A compound contains 0.50 mole of carbon for each 1 mole of hydrogen. The empirical formula of this compound is: 1) CH 2) CH2 3) C2H 4) C2H2 Example 4 The empirical formula of a compound is C2H3 and the molecular mass of the compound is 54. What is the molecular formula of the compound? Example 5 A sample of a compound contains 24 grams of carbon and 64 grams of oxygen. What is the empirical formula of this compound? Step 1 – Remember that the subscripts in a chemical formula are whole number ratios of moles. First figure out how many moles of each element we have. Step 2 –Write out the molecular formula first. Use the number of moles that you just determined as subscripts. Step 3 – Finish by converting the molecular formula to the empirical formula by dividing by the least common multiple of the subscripts. Example 6 A sample of a compound contains 156 grams of potassium and 64 grams of sulfur. What is the empirical formula of this compound? Determining Formulas from Percent Composition The empirical and molecular formulas can be determined if the % composition of the compound and the atomic masses of the elements in the compound are known. Example 1 What is the empirical formula of a compound that is 58.80% barium, 13.75% sulfur, and 27.45% oxygen by mass? Step 1 – Since all the %’s add up to 100%, let’s pretend we’re analyzing a 100 gram sample so we can convert the percentages to grams easily. 58.80% of 100 grams of barium = _______ grams of barium 13.75% of 100 grams of sulfur = _______ grams of sulfur 27.45% of 100 grams of oxygen = _______ grams of oxygen Step 2 – To determine the empirical formula, we need to know how many moles of each element there are in the compound. Step 3- We now need to determine the whole number ratio of each element in the compound. Let’s do that by dividing each # of moles by the smallest one. That guarantees the smallest number will be 1. Ba = S= O= Step 4- Let’s now write the empirical formula with the appropriate subscripts that we just determined. Example 2 What is the empirical formula of a compound that is 40% C and 6.67% H, and 53.3% O, by mass? Example 3 By chemical analysis, a molecular compound was found to consist of 80% carbon and 20% hydrogen by mass. By measuring the volume of a known mass of the compound in the gaseous phase, its molecular mass was found to be 30. Find the empirical and the molecular formulas of the compound. Example 4 Caffeine, a stimulant found in coffee, tea, and chocolate, contains 49.48% carbon, 5.15% hydrogen, 28.87% nitrogen, and 16.49% oxygen by mass, and has a molecular mass of 194.2 g/mol. Determine the molecular formula of caffeine. Example 5 Styrene is an organic molecule that is used as a building block for many polymers (like polystyrene). The molecular formula for styrene is C8H8. a. What is the molecular weight of styrene? b. What is the empirical formula of styrene? _________________ c. How much does 1.54 mole of styrene weigh? d. How many moles are in 9.67 kg of styrene? e. How many moles are in 84.6 mg of styrene? f. What is the percent composition of styrene? g. A barrel of an unknown material is recovered from the Hudson River near a plant that produces polystyrene. It has been suggested that the barrel contains styrene. Results from the elemental analysis of the unknown compound are shown below. Does this support the hypothesis that the barrel contains styrene? Element Abundance (%) Carbon 92.26 Hydrogen 7.74 h. Further experimentation determines that the molecular weight of this compound is 78.11 grams per mole. What is the molecular formula for the unknown compound?








