Unit 3 Minerals and Rocks Study Guide
advertisement

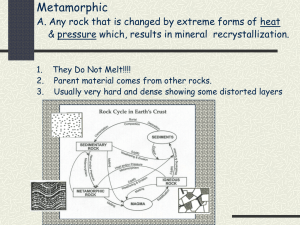
Unit 3 Minerals and Rocks Study Guide (Chapters 3, 4, 5, 7) (Revised 7/13) UNIT 3 HOMEWORK Part 1: Team written tables about minerals (see end of this unit for a guide, pages 11 - 12) Part 2: ONE WRITTEN PARAGRAPH from any selected video site: VIDEO WEB HIT HOMEWORK For any Unit Web Hits and Unit Web Videos, go to the “DMC HOME” website; in Search box –type “Geology”, select “Vernon Kramer”, scroll down to GEOL 1303, select “Syllabus”, select “Web Hit Links”, later then the select “Video Web Hits”, click on icon of interest for web sites OR: go to DMC Home website, select “Degrees, Certificates, Courses”, scroll down to Natural Sciences and select “Geology”, select “Faculty Listings”, select “Walter Vernon Kramer”, find “Geol 1303”, select “Syllabus”, and there you can find the” web hit links” click on icon of interest for web sites and then to “Web video hits”, click on icon of interest for video site [IF NONE OF THE WEB SITES COME UP, YOUR COMPUTER PROBABLY NEEDS TO BE REBOOTED (RESTARTED) - Questions: Did you complete your portion of the team written tables about minerals? Did you turn in the Unit 3 Video Hit paragraph when it was due? Some General Chemistry Terms (Chapter 3) Matter: anything that has mass and occupies space - Matter is made up of elements which are composed of atoms Element: chemical matter made up of one kind of atom Atom: the smallest unit of an element that retains the characteristics of that element An atom is composed of a nucleus of protons and neutrons, surrounded by a shell orbiting electrons (maximum 8 electrons per shell). - Rocks and minerals are natural chemicals composed of combinations of various elements (silicon iron, magnesium, carbon, oxygen, etc.). Atoms and Elements from Stars - Most of the known universe is composed of two elements: hydrogen and helium with a minor amount of all remaining elements. - Many of the remaining familiar elements are “created” within various types of stars. - The volume of the sun is composed of 92% hydrogen (H) and 7.8% helium (He) while the mass of the sun is composed of 70% H, 28% He and about 2% other elements. - Our sun is using nuclear fusion to create different elements from hydrogen and helium atoms. - Thus it is within the stars (like our sun) where smaller atoms will use nuclear fusion to create most of the dozen or so elements that we find within Earth’s rocks and minerals and atmosphere. - Most of the remaining natural (90) elements we find on Earth were created from previous supernovas (exploding massive stars that began as a mass of hydrogen and helium). We are literally made from star stuff - Carl Sagan - (Page 61 is a Periodic Table of all known elements) FYI: Our sun is a third generation star. Atoms after Supernovas - Within galaxies, most natural elements will join with others to form compounds and molecules that we see as molecular clouds (nebula) or space dust. - More than 180 compounds including methane, water, ammonia, simple amino acids and minerals have been detected within galactic nebula. 1 - This formation process is repeated throughout the known universe. Thus we should expect to find the same elements, compounds and minerals everywhere in our universe. - FYI: A molecule is the smallest unit of a substance. Example is a “single unit” of H2O or 02 (page 64) - FYI: Compound: chemical substance formed by the bonding of two or more different elements (NaCl, H2O, etc.). - (If you are interested, pages 64-66 present some details of how these atoms combine to form minerals.) Most Abundant Elements in the Earth’s Crust - The four (4) most abundant elements of the Earth’s crust (88%) include oxygen (47%), silicon (28%), aluminum (8%) and iron (5%). These same elements are easily created from a star the size of our sun. - The formation of space dust and molecules is the same for all galaxies. Thus the initial rocks and minerals on most planets and other rocky bodies should be similar to those on Earth. Concept Test Earth’s Composition - It is rocks and minerals (which are actually chemical compounds) that make up both the Earth’s crusts and the Earth’s mantle. Mineral Definition Mineral: a natural occurring inorganic solid with specific crystal structure, a specific chemical composition and with specific physical properties. (Ch. 3) All Minerals Must Possess Certain Characteristics - A mineral is a natural occurring substance that is not man-made. - A mineral is inorganic and cannot be composed of any decayed plant or animal matter. For example coal (organic) is not considered by geologists to be a mineral because it is composed of decayed organic matter. - Why are coal and oil and gas classified as minerals by the US GOVT.? – For tax purposes. Minerals are taxed at a higher rate than rocks. - A mineral must be a solid. Snow or ice is a mineral but water is not. -A mineral has a specific range of chemical compositions. The composition may be as simple as gold or complex as the mica biotite. - A mineral has a specific crystal structure. 2 Crystal: the arrangement of atoms in a solid that form in a regular, repeating three dimensional pattern - Crystal structures defined different minerals even though their chemical compositions are identical. Diamonds and Graphite (The Following Is Always Referenced on Exams) - Diamond and graphite are two different minerals both composed of the element carbon. Their differences are within their crystal structure. - Graphite (an extremely soft mineral) has a totally different crystal structure than the world’s hardest mineral (diamond). - Nothing on Earth is harder than a diamond. - A one carat diamond equals in weight to a 2” X 2” Post-it note sheet Diamonds are a girl’s best friend – Carol Channing FYI: Gem-quality diamonds can be created from the cremated remains of loved ones or pet - for a large fee. Check this out with the last listed web-hit site. - Less than 68 miles from Texas, you can dig for your own diamonds. Go to the Crater of Diamonds State Park near Murfreesboro, Arkansas for $6.50/day. You keep what you can find! Nine (9) Physical Properties of Minerals (only have to remember *3, *4 or *5 for exam) Minerals have many specific physical properties which can be used for their identification. 1. Crystal faces and shape (page 65) 2. Density (light vs. heavy) *3. Cleavage shape often taken when a minerals is broken; such as cubes of halite (pages 74) *4. Hardness resistance to abrasion, often compared to other minerals (page 75) *5. Color: color is a problem because one mineral may have many colors (page 70-71) 6. Streak; color when scratched or the color of the powdered mineral 3 7. Luster: reflected light; metallic vs. non-metallic vs. earthy (page 74) 8. Magnetism (only a few minerals are magnetic) 9. Taste (example is salt) Identifying Minerals - Identifying minerals is like identifying people. You don’t always have to use all of the characteristics to identify that person. - The only difference between rubies and sapphires is that rubies are red and sapphires are generally blue. But they are the same mineral: corundum. Get your facts first, and then you can distort them as much as you please – Mark Twain Common Texas Minerals 1. Halite (NaCl - sodium chloride) is a mineral that we all call “salt”. It is an evaporate mineral found in some dry lake beds in West Texas and sometimes along Laguna Madre. Halite is also found in large GOM salt domes where it is called rock salt. Halite is usually white to clear, forms cubic crystals (because of its atomic structure) and tastes salty. Halite has a cubic cleavage (breaks into cubes). It is used for preserving meats, french fries, Draino, leather products, de-icing roads, etc. 2. Mineral gypsum (calcium sulfate with water) is an evaporate mineral that is found in soils and sands throughout the Corpus Christi area. Gypsum has a hardness of two (2 which can be scratched with your fingernail) and has perfect cleavage (breaks into smooth sheets). It is used as a soil conditioner (especially for black-sticky soil), Plaster of Paris, Alabaster carvings and most importantly – sheetrock. 3. Mineral sulfur is an element (S) and it is also a yellow-colored mineral. Sulfur is commonly found in the cap rock of salt domes and near steam vents that surround volcanoes. Trace amounts of sulfur are essential for life but large quantities can be toxic. Sulfur is used for gunpowder, medicine, and agriculture and during the refining of gasoline. Rotten-egg smell is produced by sulfur dioxide. In ancient times, sulfur was called brimstone. 4. Mineral calcite (calcium carbonate - CaCO3) cleaves (breaks) itself into a rhombic pattern. This creates the rocks limestone and caliche. Massive quantities of calcite rocks are found throughout Texas as large deposits of caliche (found in the Mathis area). (Pits are seen alongside the highway.) 4 5. Mineral magnetite (Fe3O4) is a heavy, hard, black mineral that is a natural magnet. It is commonly found in beach and river sand and deposits in Central Texas. Magnetite is the principle mineral of iron ore. Silicate Minerals (pages 67-69) - 75% of the Earth’s mass is made up of silicon and oxygen. - Silicate minerals (minerals with some silicon & oxygen) are the most common minerals on Earth. They form more than 1/3 of all known minerals -Silicate minerals comprise 95% of the volume of the Earth’s crust. 6. Silicate mineral quartz (silicon dioxide SiO2) is very hard. Quartz can scratch glass. But steel cannot scratch quartz. Quartz in CC area includes beach and river sands, and chert (flint or gravel). Quartz gemstones include amethyst, agate, and rose quartz. Melted quartz sand is used to make glass. Sand and chert are among the most important components of concrete used with the building industry. 7. The feldspar group of silicates includes orthoclase. Orthoclase has a rectangular cleavage. Orthoclase is used in making ceramics. We will most often notice orthoclase as the “salmoncolored” mineral in granite. 8. The mica group silicates include biotite (black) and muscovite (clear). All have planar cleavage and break into very thin flexible sheets. Muscovite is used for electrical insulators and glitter. Biotite is one of the black minerals that we find within granite. 9. Olive-green olivine is one of the most common mineral in the known universe. Clear olivine is often cut into the gemstone peridot. Where Do Minerals Form? - Minerals can form in many environments including in space as galactic nebula and molecular clouds. - On Earth, minerals can be formed by many methods: 1. From the cooling of magmas and lavas 2. From evaporation of mineralized waters or fluids 3. From reactions with natural acid waters 4. As a byproduct of living organisms: grass (quartz), sea plants and sea animals (quartz and calcite), and animals (mineral apatite in bones and teeth) Rocks Rock: a naturally occurring aggregate of one or more minerals. 5 Diplomacy is the art of saying “Nice Doggie” until you can find a rock – Will Rogers Creation of Rocks (pages 17-18) - Rock can be formed from many processes: 1. A rock may be a solid made from a combination of minerals. 2. A rock might be a very large body or deposit made of a single mineral (halite vs. salt). 3. A rock may be solidified organic matter formed by biochemical processes (coal, peat, etc.). 4. A rock composed of quartz or calcite can be created by biochemical processes (chert and limestone). - Minerals make up most rocks. Rock Systems - Rocks are formed in three geological systems: 1) Igneous System rocks; 2) Sedimentary System rocks and 3) Metamorphic System rocks. - Each system contains a collection of different rock names and types. - The rock types differ from each other by size, shape and arrangement of the mineral grains. - Rocks can be modified by different geological environments (systems) – for example sedimentary rock sandstone can be melted to become an igneous rock. Those are my principles, and if you don’t like them… well, I have others – Groucho Marx Rock Cycle - All three systems of rocks are interrelated with each other and are part of a “rock cycle” (BE ABLE TO DEFINE from a model) Rock cycle: (page 18) a general model that describes how various geologic processes can create and modify rocks. (A different drawing of a rock cycle drawing is found at the end of this study guide.) - Plate tectonics and the hydrologic system are responsible for the recycling of rock material and are the driving forces that can take hundreds of millions of years to completely change a rock. The Rock Cycle Concept Test 6 Igneous Rock System Definitions (Ch. 4 & 5) IGNEOUS ROCK: a rock solidified from a molten state MAGMA: molten rock within the Earth’s surface LAVA: a molten rock (magma) that has reached the Earth’s surface - If the rock is melted – it is a magma or lava. If the rock is no longer melted and is solid – it’s an igneous rock. EXTRUSIVE ROCKS: an igneous rock that formed on the Earth’s surface from the cooling of lava; often referred to as volcanic rocks; generally has some gas bubbles INTRUSIVE ROCKS: an igneous rock that formed from the cooling of magma, under the surface of the Earth; generally has visible crystals Some Locally Found Igneous Rocks Scoria: A low density, dark-colored mafic extrusive igneous rock containing abundant gas vesicles (exploded from a volcano) - Scoria is usually black but acid gasses can cause the scoria to be red. Scoria is used for BBQ pits and decorative rocks Pumice: A low density, light-colored felsic extrusive igneous rock containing abundant gas vesicles that was exploded from a volcano. - Pumice is the only igneous rock that floats on water and is commonly found on Padre Island. - Pumice is often sold for foot care and is used for abrasion. Granite: a coarse-grained, light-colored, felsic intrusive igneous rock composed of Biotite, Orthoclase (Feldspar) and Quartz - The nearest Texas granite mass is the Enchanted Rock, NW of San Antonio. - Large blocks of granite are used for a jetty at Port Aransas. Sediments (Ch. 7) - There must first be a sediment before there can be a sedimentary rock. Sediment: unconsolidated (loose) earth material, with sizes that range from gravel to mud; would include any natural substance transported and deposited by wind, water, ice and gravity 7 Two General Classifications of “Sediments” (needed to make sedimentary rocks): 1. Clastic or Detrital sediments: solid particles derived from pre-existing rocks 2. Chemical sediments A. Biochemical sediments: solid particles created by plants and animals B. Evaporation sediments: solid particles derived from evaporation Sediments Form Layers of Sediments that Eventually Become Sedimentary Rocks - Sediments are deposited into layers which will compact to form rock layers. Strata: distinct layers of sediments or rocks Formation: a major, widespread unit of sediment or rock (many strata) that is different from the rocks above or below the unit Lithification: a process of compaction and/or cementation that changes sediments into a rock The Different Classifications of Sediments Produce Different Types of Sedimentary Rocks 1. Clastic or Detrital Sedimentary Rocks: Rocks that are consolidated from clasts (pieces) of other rocks Sandstone: rock made of consolidated sand-size particles Conglomerate: rock made from consolidated sand and gravel sized particles Cementation is accomplished by precipitation of minerals within sediments (coating with minerals of calcite, silica or iron) 2. Chemical Sedimentary Rocks A. Rocks that are consolidated from biochemical processes of vegetation Coal: vegetation matter modified by biochemical processes and consolidated into a rock - Coal is burned for electricity production (50% of US electricity) and for making chemicals and cosmetics - FYI: Human bodies thrown into swamps and peat bogs can turn into coal – Google “peat bog man” 8 B. Rocks derived from skeletons of microscopic plants and animals Limestone: a carbonate rock (calcite) consolidated from muds of plant and animal skeletons - Large areas of Texas are underlain with limestone - Limestone commonly contains fossil sea shells and sea animal fossils - Limestone has removed huge quantities of carbon dioxide from the atmosphere. C. Rocks derived from consolidated muds - Another rock that is big in South Texas is shale which is basically consolidated, harden mud, that can be rich with organic material and this includes the Eagle Ford shale - The Eagle Ford shale has led to a major oil and gas boom in south Texas - All of this is possible because of direction drilling and fracturing or fracing with sand and water to open up the rock so that the oil/gas can escape into the pipes. D. Rocks that are consolidated from evaporation and/or precipitation Caliche: a “powdery” type of “evaporative” limestone found throughout South Texas, especially near Mathis; used for road construction Concept Test Nature does nothing uselessly - Aristotle Metamorphic System Rocks (Ch. 8) - Rocks formed from pre-existing rocks that have been subjected to great heat and pressure - Metamorphic rocks often appear squashed and shiny Metamorphism: the original material (rocks) has been changed (recrystallized) because of great heat and/or great pressure into a new appearance (same material) - There are two classifications of metamorphic rocks (and both types are found in Central TX and West TX) 9 1. Foliated (layered): example is Gneiss: (metamorphosed granite) a coarse grained metamorphic rock with alternating light and darker colors (foliated) 2. Nonfoliated (not layered): example is Marble: metamorphosed limestone can be found at Marble Falls, TX (NW of San Antonio). - Remember that limestone is composed of the mineral – calcite! SEE PAGES 11 – 12 FOR INSTRUCTIONS FOR RESEARCH PAPER Consider having the Stone Writing Center review your paper 10 Teamwork Research – (Two Parts) Rocks and Minerals Unit 3 Any advanced society must use rock and minerals and metals derived from minerals. And these substances much be taken from the Earth (mined) in order to use them. Our society can only exist because of mining for minerals somewhere on Earth. As our society becomes more advanced, we use a wider range of metals and minerals for almost everything in our lives. Thus recycling of minerals and metals takes on a greater importance to our society. But what minerals and metals are we using? Your Team assignment is to select one of four categories of a list of items to do your research. You are to search numerous web sites and make a list of minerals and metals used (suggested form given below). You are to make copies of your list for your teammates and each student is to discuss within the group their findings. One thing you will discover is our increasing use of REE or Rare Earth Elements which is hard to find and extract. PART 1: Below are the 4 categories available (a different one for each team member): A. An automobile (at least 30 elements and metals and minerals)(list 2 websites used) B. A computer including all its chips (more than 60 elements, metals and minerals)(list 2 websites used) C. Cell phones of various types (more than 38 elements, metals and minerals) (list 2 websites used) D. Various types of light bulbs such as incandescent. CFL, neon lights (more than 20 minerals, metals and gasses) (list 2 websites used) General rules: 1. To be typed: 2. Column 1: with the metals, etc. in alphabetical order; 3. Column 2 are the host minerals (from possibly a mineral website) that matches the metal or element. 4. If the website does not list a metal but a mineral instead, put its name in the metal column Example: Metal/Element 1. Iron 2. Lead 3. Etc. Mineral magnetite galena etc. Websites used: http://www.mii.org/pdfs/cfl-bulbs.pdf http://webmineral.com/Mineral_Definition.shtml Form to use can be a word document or a spreadsheet. INDIVIDUALLY: WORTH FIVE (5) POINTS for your research paper, IF TURN IN ON TIME NOT ACCEPTED LATE and 2 points for team work, if not late Part 2: TEAMWORK GROUP worth an extra 2 points for each participating member: 1. Using all of the individual research, turn in one list that includes the same 10 elements or minerals found in each of the other lists 2. Turn in your list and common mineral list to me See page 14 Unit 2 GOM Communication Skills for example and grades of writing rubric SEE GRADING RUBRIC FOR TEAMWORK (NEXT) PAGE 12 11 ALL WORK (INCLUDING TEAM WORK) IS DUE CLASS AFTER EXAM 2 (Monday) TEAMWORK SKILLS Aspect 2: Be able to work effectively with others to support a shared purpose or goal. I will assign a zero (0) to any work sample that does not meet Benchmark Expectations. Exceeds Target Expectations 2 points Meets Target Expectations 1 points Contributes to Team Meetings Advances the work of the group by a. achieving Target Expectations AND b. articulating the merits of alternative ideas or proposals. Individual Contributions outside of Team Meetings a. Achieves Target Expectations and at a. Achieves Benchmark Expectations least one of the following AND b. Work accomplished advances b. Work accomplished is thorough the project. and comprehensive. Fosters Constructive Team Climate Advances the work of the group by a. offering new suggestions to advance the work of the group AND/OR b. offering alternative solutions or courses of action that build on the ideas of others. Below Benchmark Expectations 0 points Shares ideas but does not advance the work of the group Completes all assigned tasks by a deadline. c. Proactively helps other team members complete their assigned tasks to a level of excellence. Supports a constructive team climate by doing at least three of the following: a. Treats team members respectfully by being polite and constructive in communication. b. Uses positive vocal or written tone, facial expressions, and/or body language to convey a positive attitude about the team and its work. c. Motivates teammates by expressing confidence about the importance of the task and the team’s ability to accomplish it. d. Provides assistance and/or encouragement to team members. Supports a constructive team climate by Supports a constructive team climate by doing two of the following: doing one of the following: a. Treats team members a. Treats team members respectfully by being respectfully by being polite and polite and constructive in communication. constructive in communication. b. Uses positive vocal or written tone, facial b. Uses positive vocal or written expressions, and/or body language to convey tone, facial expressions, and/or a positive attitude about the team and its body language to convey a positive work. attitude about the team and its c. Motivates teammates by expressing work. confidence c. Motivates teammates by about the importance of the task and the expressing confidence about the team’s ability to accomplish it. importance of the task and the d. Provides assistance and/or encouragement to team members. team’s ability to accomplish it. d. Provides assistance and/or encouragement to team members. 12