THE WORKING CELL (CH 5) TRANSPORT OF LARGE MOLECULES
advertisement

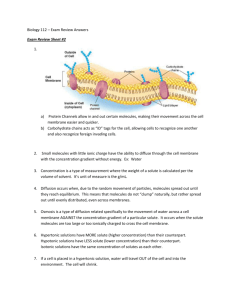
9/2/2015 THE WORKING CELL (CH 5) NUCLEUS Nuclear envelope Nucleolus Rough endoplasmic reticulum CYTOSKELETON Microtubule Microfilament Intermediate filament Ribosomes Smooth endoplasmic reticulum Plasma membrane Golgi apparatus Lysosome Mitochondrion Centrosome with pair of centrioles TRANSPORT OF LARGE MOLECULES Endocytosis transport large molecules Phagocytosis “cell eating” • Engulf the food • Package in a membrane enclosed vacuole • Lysosomes digest it! Pseudopodium “Food” or other particle Food vacuole Receptor Specific molecule Coated pit Receptor mediated endocytosis • Receptor proteins • Package in a membrane enclosed vacuole • Lysosomes digest it! 1 9/2/2015 TRANSPORT OF LARGE MOLECULES Exocytosis and endocytosis transport large molecules Exocytosis “exports” large molecules 1. Molecule in a membrane enclosed vesicle moves from Golgi apparatus to membrane 2. Vesicle fuses with plasma membrane 3. Molecules spill out of cell CELL MEMBRANES Small molecules and ions move across membranes or through protein channels. 2 9/2/2015 DIFFUSION The tendency of a particle to move along a concentration gradient DIFFUSION The tendency of a particle to spread out into available space (along a concentration gradient) Molecules of dye Membrane Pores Net diffusion Net diffusion Equilibrium FIG 5.3A 3 9/2/2015 PASSIVE TRANSPORT • Diffusion of molecules across a cellular membrane • Requires no energy CO2 O2 FACILITATED DIFFUSION Diffusion of polar molecules with the help of transport proteins Polar molecule • Specific transport proteins for specific types of molecules • Aquaporins transport water across cell membranes Transport protein FIG 5.6 4 9/2/2015 WATER TRANSPORT IN A CELL Think about a cell full of pure water, in a cup of pure water. Easy right? H2O H2O WHAT IF THE CELL IS IN A SOLUTION? Solution: a liquid consisting of a uniform mixture of two or more substances • SOLUTION = Solute(s) + solvent • SOLUTE= the substance that is dissolved • SOLVENT= the dissolving agent 5 9/2/2015 TABLE SALT IN WATER Na Na Sodium atom Cl • • • • Salts are formed by ionic bonds Strong in air Bonds break in water Form Na+ and Cl‐ ions Cl Chlorine atom Cl− Na+ FIG 2.7 A,B WATER TRANSPORT IN CELLS • In a solution, water molecules bond with solute molecules • Creates a gradient of “free” water molecules (Diffusion is the tendency of a particle to move along a concentration gradient) 6 9/2/2015 WATER TRANSPORT IN CELLS • Water molecules can move across aquaporin channels but solute molecules cannot. • A gradient of “free” water molecules is created • Water molecules move along the concentration gradient of “free molecules” OSMOSIS The diffusion of water across a SELECTIVELY permeable membrane 7 9/2/2015 TYPES OF SOLUTIONS TONICITY ‐ the ability of a solution to cause a cell to gain or lose water Less solutes outside than inside cell Solutes are the same inside and outside cell More solutes outside than inside cell CELL WATER BALANCE IN SOLUTIONS Hypotonic solution H2O Lysed Isotonic solution Hypertonic solution H2O H2O Normal H2O Shriveled 8 9/2/2015 WATER BALANCE Cell walls prevent lysing in hypotonic solutions Hypotonic solution Isotonic solution Plasma membrane H2O H2O Lysed Hypertonic solution Normal Flaccid Turgid (normal) H2O Shriveled Shriveled (plasmolyzed) Osmoregulation: an organism’s control of water balance WATER BALANCE Cell walls prevent lysing in hypotonic solutions Hypotonic solution Isotonic solution Plasma membrane H2O H2O Lysed Turgid (normal) Hypertonic solution Normal Flaccid H2O Shriveled Shriveled (plasmolyzed) Osmoregulation: an organism’s control of water balance 9 9/2/2015 MOVEMENT ACROSS MEMBRANES Passive transport 1. Non polar molecules pass across membranes via DIFFUSION O2 CO2 2. Polar molecules pass across specific channel proteins via FACILITATED DIFFUSION 3. Water molecules pass along aquaporins via FACILITATED DIFFUSION and transport is affected by the process of OSMOSIS H2O 10 9/2/2015 MOVEMENT ACROSS MEMBRANES Active transport: moving a solute AGAINST the concentration gradient Transport protein • Active transport proteins move molecules in or out of cells AGAINST the concentration gradient • Specific transport proteins for specific molecules Requires ENERGY Solute 11 9/2/2015 ACTIVE TRANSPORT Active transport: moving a solute AGAINST the concentration gradient Transport protein Energy Solute 1 Solute binds to transport protein. 2 Energy is added to change the protein shape. 3 Protein returns to original shape and more solute can bind. ENERGY The capacity to cause change or to perform work 1. Kinetic Energy: the energy of motion • Thermal energy (heat) – energy of atoms vibrating back and forth • Sound • Electricity • Light 2. Potential Energy: energy resulting from location or structure • Chemical • Gravitational 12 9/2/2015 CHEMICAL ENERGY Cells use potential chemical energy to perform work Potential energy is the energy matter pocesses as a result of it’s location or structure ENERGY TRANSFORMATION Laws of thermodynamics 1. Energy can not be created or destroyed 2. Energy transforms increase entropy of the universe 13 9/2/2015 ENERGY TRANSFORMATION Laws of thermodynamics 2. Energy transforms increase entropy of the universe • Energy is given off as heat in each energy transfer • The original energy becomes more disorganized (i.e. entropy increases) at each step. THURSDAY Details of how energy is transferred and renewed in cells End of CH4, beginning of CH5 • Requires ENERGY 14 9/2/2015 TODAY’s CHECK‐IN (3pts) Open Notebook & NOTHING ELSE! 1. If an animal cell containing 2% of polar molecule “A” is put into a solution containing 50% of molecule “A”, based on what we know about osmosis, what will happen to water molecules inside the animal cell? (1pt) 2. What if it is a plant cell? (1pt) . 3. RATE YOUR UNDERSTANDING A) Easy money B) I should probably review passive transport C) This was hard, I should probably stop by for office hours to clarify. REQUIRED VIDEO This core concepts of this material is fair game for our next check‐in AND ALL EXAMS! ALL OF THE ENERGY OF THE UNIVERSE IS…. See link on the course website: http://www.biosbcc.net/harrer 15