E - Bio @ Horton AP Biology
advertisement

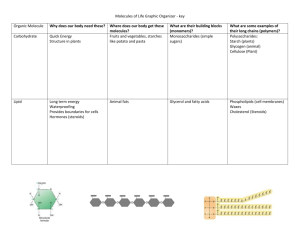
E - Bio @ Horton Unit – Biochemistry Notes – Intro Biochem AP Biology Carbon and Its Role in Macromolecules A. Definitions 1. 2. 3. 4. Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. Chemistry of carbon allows the formation of an enormous variety of organic molecules. Organic molecules have carbon bonded to other atoms and determine structure and function of living things. 5. Inorganic molecules do not contain carbon and hydrogen together; inorganic molecules (e.g., NaCl) can play important roles in living things. B. Carbon Skeletons and Functional Groups 1. Carbon has four electrons in outer shell; bonds with up to four other atoms (usually H, O, N, or another C). 2. Ability of carbon to bond to itself makes possible carbon chains and rings; these structures serve as the backbones of organic molecules. 3. Functional groups are clusters of atoms with characteristic structure and functions. a. Polar molecules (with +/- charges) are attracted to water molecules (hydrophilic). b. Nonpolar molecules are repelled by water and do not dissolve in water; these are hydrophobic. c. Hydrocarbon is hydrophobic except when it has an attached ionized functional group such as carboxyl (acid) (--COOH); then molecule is hydrophilic. d. Cells are 70-90% water; the degree organic molecules interact with water affects their function. Notes Biochemistry 1 4. Isomers are molecules with identical molecular formulas but differ in arrangement of their atoms. C. Building Polymers 1. Four classes of macromolecules (polysaccharides, triglycerides, polypeptides, and nucleic acids) provide great diversity. 2. Small organic molecules (e.g., monosaccharides, glycerol and fatty acid, amino acids, and nucleotides) serve as monomers, the subunits of polymers. 3. Polymers are the large macromolecules composed of three to millions of subunits. D. Condensation and Hydrolysis 1. Macromolecules and large polymer molecules. 2. Macromolecules build by different bonding of different monomers; mechanism of joining and breaking these bonds is condensation and hydrolysis. Notes Biochemistry 2 3. Cellular enzymes carry out condensation and hydrolysis of polymers. 4. During condensation synthesis, a water is removed (condensation) and a bond is made (synthesis). a. When two monomers join, a hydroxyl (--OH) group is removed from one monomer and a hydrogen is removed from the other. b. This produces the water given off during a condensation reaction. 5. Hydrolysis reactions break down polymers in reverse of condensation; a hydroxyl (--OH) group from water attaches to one monomer and hydrogen (--H) attaches to the other. E. Functional Groups Specific chemical properties are a result of the presence of particular functional groups. Functional groups are clusters of atoms with characteristic structure and functions. A functional group is a portion of a molecule which reacts in a particular way. The marvelous thing about them is that their chemistry is largely independent of what else is on the same molecule! Thus an -OH group will react pretty much the same whether it is attached to a single -CH3 group or a carbon chain which has thousands of atoms in it. Images: Campbell, Neil and Reece, Jane. Biology (6th ed.) San Francisco: Benjamin Cummings Modified Notes: Mader, Sylvia. Biology (7th ed) New York: McGraw Hill Publishing Notes Biochemistry 3