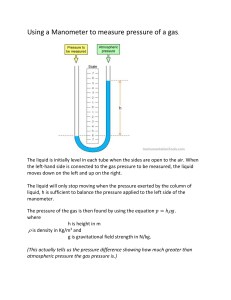
manometer

Aim:
Measure normal atmospheric pressure
Explanation of theory:
A manometer is an device employed to measure pressure. A simple, common design is to seal a length of glass tubing and bend the glass tube into a U-shape. The glass tube is then filled with a liquid so that all trapped air is removed from the sealed end of the tube. The glass tube is then positioned with the curved region at the bottom.
After the liquid settles to the bottom of the manometer, a vacuum is produced in the sealed tube (the left tube in the picture). The open tube is connected to the system whose pressure is being measured(the atmosphere). In the sealed tube, there is no gas to exert a force on the liquid. In the tube connected to the system, the gas in the system exerts a force on the mercury. The net result is that the column of liquid in the sealed tube is higher than that in the unsealed tube. The difference in the heights of the columns of mercury is a measure of the pressure of gas in the system.
The force exerted by the column of liquid in a tube arises from the gravitational acceleration of the liquid, Newton's Second Law: F = m g
Hence, we can obtain: m g
P =
A
Materials & Apparatus:
Manometer
Water
Metre rule
Retort stand
Procedure:
1.
Measure the diameter of the manometer’s opening
2.
Add 20ml of water into the unsealed side of the manometer.
3.
Tilt the manometer such that the water fills up the entire column of the seal side
4.
Connect the manometer to the retort stand.
5.
Measure the height of the liquid on both unseal and seal sides
6.
Repeat steps 2-5 to ensure accuracy of experiment
7.
Calculate the average result and the pressure using the equation shown above.
(Assume g to be 9.81 and density of water to be 1)
References:
http://ag.arizona.edu http://www.chm.davidson.edu http://www.kentchemistry.com http://www.ehow.com