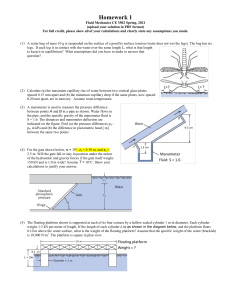
Using a Manometer to measure pressure of a gas
.
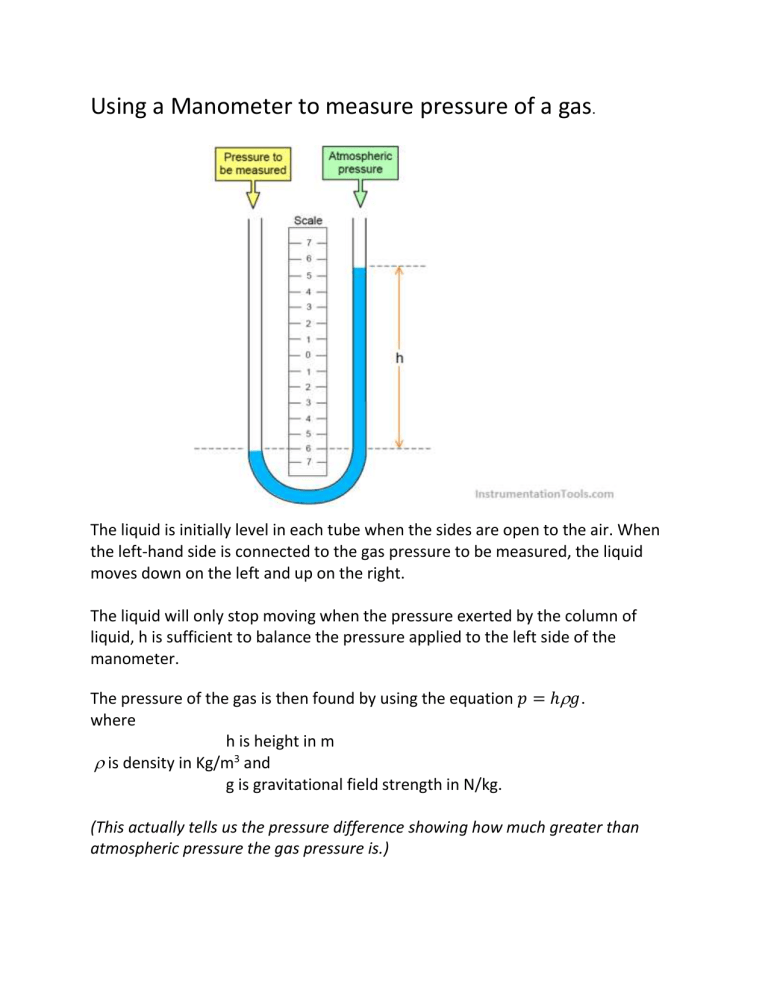
The liquid is initially level in each tube when the sides are open to the air. When the left-hand side is connected to the gas pressure to be measured, the liquid moves down on the left and up on the right.
The liquid will only stop moving when the pressure exerted by the column of liquid, h is sufficient to balance the pressure applied to the left side of the manometer.
The pressure of the gas is then found by using the equation 𝑝 = ℎ
𝑔 . where h is height in m
is density in Kg/m 3 and g is gravitational field strength in N/kg.
(This actually tells us the pressure difference showing how much greater than atmospheric pressure the gas pressure is.)
Question:
A manometer can be used to find the pressure of a gas. The difference in pressure in the level of the liquid in each side of the tube indicates the pressure of the gas. gas a) What is the difference in pressure between A and b? Use g = 10N/kg b) What is the pressure of the gas in tube C? c) Explain why this pressure is the pressure of the gas above atmospheric pressure.