B will check them
advertisement

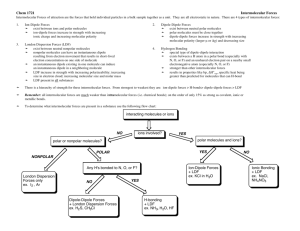
Homework • Page 8 – 1st column for #1 and #2 • Correct lab and resubmit Lab • Everyone can improve their grade! • Answer any questions you got wrong on a sheet of NOTEBOOK paper (not in lab notebook) • Staple to the back of the original lab • Return next class • Use notes from today (in addition to POGIL and page 2 notes) in order to help you So you know… • Bonds and forces are different • NO BONDS WERE BROKEN IN THIS LAB Page 2 • Let’s update our notes • Look at the strength row – Strength of DD depends on _____ – Strength of LDF depends on _____ So you know… • The number of atoms in a molecule would only be important if we were talking about LDF – More atoms would mean more electrons With your face partner, finish your assigned sentence B. Strength of LDF depends on….. A. Strength of DD depends on…. With your face partner, finish your assigned sentence B. Strength of LDF depends on the number of electrons (more electrons means a stronger LDF) A. Strength of DD depends on the polarity of the molecule which depends on the difference in electronegativity (larger difference, more polar, stronger DD) Intra vs Inter Reminder • Bottom of pg 2 The state a substance is in depends on the strength of the intermolecular forces States of Matter • Solid • Liquid • Gas • Plasma Make this chart in your notes (5 columns, 6 rows) solids Kinetic energy Shape Volume Intermolecular forces picture liquids Gases Plasma Solids – very low KE - particles vibrate but can’t move around – fixed shape – fixed volume – molecules are very close together – Very strong attractive forces between molecules • Be specific about the ‘force’ type if you are given a specific substance (LDF, DD, etc.) Liquids – low KE - particles can move around but are still close together – variable shape – fixed volume - atoms and molecules are touching but can move throughout container – strong attractive forces between molecules Gases – high KE - particles can separate and move throughout container – variable shape – variable volume - molecules are moving in random patterns with varying amounts of distance between the particles. – NO attractive forces between molecules Plasma – very high KE - particles collide with enough energy to break into charged particles (+/-) – gas-like, variable shape & volume – stars, fluorescent light bulbs, CRTs – NO attractive forces You are a molecule • What would it look like if…. Requires adding energy in order to break the attractive forces between the molecules. Remember • Nonpolar molecules have LDF • Polar molecules have LDF and DD – If the polar molecule has H attached to nitrogen, oxygen, or fluorine, the substance has LDF, DD, and HB Example Question & Answer The normal boiling point of CCl4 is 77°C, whereas that of CBr4 is 190°C. Explain. Mention specific IMFs for BOTH substances Both CCl4 and CBr4 are nonpolar. This means that Explain why London dispersion forces hold the molecules togetherthe strengths in the liquid state. To boil the substances, the LDF of the forces different must be broken. Since CBr4 has more electrons than CCl4, a stronger momentary dipole can be formed when its electrons shift. Therefore, the molecules are more attracted to each other and more energy is required to break those attractive LD forces. Make claim to answer question Your Turn • Mention specific IMFs for BOTH substances • Explain why the strengths of the forces different • Make claim to answer question • Ammonia, NH3, has a significantly higher boiling point than PH3. Both substances are trigonal pyramidal. Explain. • Ammonia is a polar molecule that has LDF, dipole-dipole, and hydrogen bonds. PH3 is also polar; it has LDF and dipole-dipole attractions. Since ammonia has an additional strong type of intermolecular force, its boiling point will be higher. • • • • Ionic Nomenclature Must be neutral overall NO PREFIXES!!!!!! Name the cation first, then the anion Cation is either a metal or ammonium – To name a metal cation, use the element name and a roman numeral to indicate the charge of the cation • • • • Tin(II) is Sn2+ Tin(III) is Sn3+ Mercury(II) is Hg2+ Mercury(I) is Hg22+ (it’s diatomic) – Roman numerals are NOT used for the following because they only have one charge: Group 1A (+1), Group 2A(+2), ammonium(+1), or CdZnAgAl 2213 (where 2 is the charge of Cd, 2 is the charge of Zn, etc.) • Sodium is Na+ • Magnesium is Mg2+ • Cadmium is Cd2+ Ionic Nomenclature • Anion is either a nonmetal or a negative polyatomic ion (like sulfate or phosphite) • Use the ion name for the nonmetal (Cl- is chloride) • Some examples for you to look at before we practice • Zr(NO3)2 = zirconium(II) nitrate – Since nitrate has a -1 charge and there are 2 of them, zirconium must have a +2 charge to make the compound neutral overall • CuCl = copper(I) chloride • CuCl2 = copper(II) chloride – the # of Cl- depends on the charge of Cu ion • Ag3PO4 = silver phosphate (no roman numerals because this is part of CdZnAgAl 2213) Quiz-Quiz-Trade • What’s my roman numeral? Work with your shoulder partner – A does #1 and B checks, B does #1 and A checks A does #2 and B checks, etc. the answers will be displayed when everyone is done These are A’s problems (B will check them) These are B’s problems (A will check them) 1. 2. 3. 4. 1. 2. 3. 4. Au(ClO)2 MgO Fe2S3 AlN If there are groups of 3, just rotate A, B, and C CoP Ag2CO3 Ca(CN)2 PbF2 Hint: each set has 2 that need roman numerals and 2 that do not Answers These are A’s problems (B will check them) These are B’s problems (A will check them) 1. Au(ClO)2 gold(II) hypochlorite 2. MgO magnesium oxide 3. Fe2S3 iron(III) sulfide 4. AlN aluminum nitride 1. CoP cobalt(III) phosphide 2. Ag2CO3 silver carbonate 3. Ca(CN)2 calcium cyanide 4. PbF2 lead(II) fluoride 1. 2. 3. 4. Silver hypochlorite Iron(II) cyanide Calcium nitride Nickel(I) phosphate Work these on your own – 1. 2. 3. 4. 5. (but ask your partner if you need help) Careful: I mixed in some covalent…. FeSO4 1. iron(II) sulfate (NH4)2CrO4 2. ammonium chromate NI3 3. nitrogen triodide Fe(HCO3)3 4. iron (III) bicarbonate Co(C2H3O2)3 5. Cobalt(III) acetate Exit Ticket 1. Y(OH)2 2. FeBr3 3. ZnSO3 4. Platinum(IV) phosphate 5. Tungsten(VI) cyanide 6. Cadmium oxalate